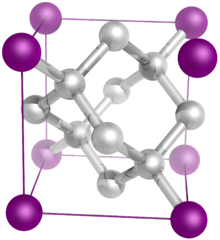
- Crystal system
-
In crystallography, the terms crystal system, crystal family, and lattice system each refer to one of several classes of space groups, lattices, point groups, or crystals. Informally, two crystals tend to be in the same crystal system if they have similar symmetries, though there are many exceptions to this.
Crystal systems, crystal families, and lattice systems are similar but slightly different, and there is widespread confusion between them: in particular the trigonal crystal system is often confused with the rhombohedral lattice system, and the term "crystal system" is sometimes used to mean "lattice system" or "crystal family".
Space groups and crystals are divided into 7 crystal systems according to their point groups, and into 7 lattice systems according to their Bravais lattices. Five of the crystal systems are essentially the same as five of the lattice systems, but the hexagonal and trigonal crystal systems differ from the hexagonal and rhombohedral lattice systems. The six crystal families are formed by combining the hexagonal and trigonal crystal systems into one hexagonal family, in order to eliminate this confusion.
Contents
Overview
Hexagonal Hanksite crystal, with three-fold c-axis symmetry
A lattice system is a class of lattices with the same point group. In three dimensions there are seven lattice systems: triclinic, monoclinic, orthorhombic, tetragonal, rhombohedral, hexagonal, and cubic. The lattice system of a crystal or space group is determined by its lattice but not always by its point group.
A crystal system is a class of point groups. Two point groups are placed in the same crystal system if the sets of possible lattice systems of their space groups are the same. For many point groups there is only one possible lattice system, and in these cases the crystal system corresponds to a lattice system and is given the same name. However, for the five point groups in the trigonal crystal class there are two possible lattice systems for their point groups: rhombohedral or hexagonal. In three dimensions there are seven crystal systems: triclinic, monoclinic, orthorhombic, tetragonal, trigonal, hexagonal, and cubic. The crystal system of a crystal or space group is determined by its point group but not always by its lattice.
A crystal family also consists of point groups and is formed by combining crystal systems whenever two crystal systems have space groups with the same lattice. In three dimensions a crystal family is almost the same as a crystal system (or lattice system), except that the hexagonal and trigonal crystal systems are combined into one hexagonal family. In three dimensions there are six crystal families: triclinic, monoclinic, orthorhombic, tetragonal, hexagonal, and cubic. The crystal family of a crystal or space group is determined by either its point group or its lattice, and crystal families are the smallest collections of point groups with this property.
In dimensions less than three there is no essential difference between crystal systems, crystal families, and lattice systems. There are 1 in dimension 0, 1 in dimension 1, and 4 in dimension 2, called oblique, rectangular, square, and hexagonal.
The relation between three-dimensional crystal families, crystal systems, and lattice systems is shown in the following table:
Crystal family Crystal system Required symmetries of point group point groups space groups bravais lattices Lattice system Triclinic None 2 2 1 Triclinic Monoclinic 1 twofold axis of rotation or 1 mirror plane 3 13 2 Monoclinic Orthorhombic 3 twofold axes of rotation or 1 twofold axis of rotation and two mirror planes. 3 59 4 Orthorhombic Tetragonal 1 fourfold axis of rotation 7 68 2 Tetragonal Hexagonal Trigonal 1 threefold axis of rotation 5 7 1 Rhombohedral 18 1 Hexagonal Hexagonal 1 sixfold axis of rotation 7 27 Cubic 4 threefold axes of rotation 5 36 3 Cubic Total: 6 7 32 230 14 7 Crystal systems
The distribution of the 32 point groups into the 7 crystal systems is given in the following table.
crystal family crystal system point group / crystal class Schönflies Hermann-Mauguin Orbifold Coxeter Type order structure triclinic triclinic-pedial C1 1 11 [ ]+ enantiomorphic polar 1 trivial triclinic-pinacoidal Ci 1 1x [2,1+] centrosymmetric 2 cyclic monoclinic monoclinic-sphenoidal C2 2 22 [2,2]+ enantiomorphic polar 2 cyclic monoclinic-domatic Cs m *11 [ ] polar 2 cyclic monoclinic-prismatic C2h 2/m 2* [2,2+] centrosymmetric 4 2×cyclic orthorhombic orthorhombic-sphenoidal D2 222 222 [2,2]+ enantiomorphic 4 dihedral orthorhombic-pyramidal C2v mm2 *22 [2] polar 4 dihedral orthorhombic-bipyramidal D2h mmm *222 [2,2] centrosymmetric 8 2×dihedral tetragonal tetragonal-pyramidal C4 4 44 [4]+ enantiomorphic polar 4 Cyclic tetragonal-disphenoidal S4 4 2x [2+,2] 4 cyclic tetragonal-dipyramidal C4h 4/m 4* [2,4+] centrosymmetric 8 2×cyclic tetragonal-trapezoidal D4 422 422 [2,4]+ enantiomorphic 8 dihedral ditetragonal-pyramidal C4v 4mm *44 [4] polar 8 dihedral tetragonal-scalenoidal D2d 42m or 4m2 2*2 [2+,4] 8 dihedral ditetragonal-dipyramidal D4h 4/mmm *422 [2,4] centrosymmetric 16 2×dihedral hexagonal trigonal trigonal-pyramidal C3 3 33 [3]+ enantiomorphic polar 3 cyclic rhombohedral S6 (C3i) 3 3x [2+,3+] centrosymmetric 6 cyclic trigonal-trapezoidal D3 32 or 321 or 312 322 [3,2]+ enantiomorphic 6 dihedral ditrigonal-pyramidal C3v 3m or 3m1 or 31m *33 [3] polar 6 dihedral ditrigonal-scalahedral D3d 3m or 3m1 or 31m 2*3 [2+,6] centrosymmetric 12 dihedral hexagonal hexagonal-pyramidal C6 6 66 [6]+ enantiomorphic polar 6 cyclic trigonal-dipyramidal C3h 6 3* [2,3+] 6 cyclic hexagonal-dipyramidal C6h 6/m 6* [2,6+] centrosymmetric 12 2×cyclic hexagonal-trapezoidal D6 622 622 [2,6]+ enantiomorphic 12 dihedral dihexagonal-pyramidal C6v 6mm *66 [6] polar 12 dihedral ditrigonal-dipyramidal D3h 6m2 or 62m *322 [2,3] 12 dihedral dihexagonal-dipyramidal D6h 6/mmm *622 [2,6] centrosymmetric 24 2×dihedral cubic tetrahedral T 23 332 [3,3]+ enantiomorphic 12 Alternating tetrahedral Td 43m *332 [3,3] 24 symmetric diploidal Th m3 3*2 [3+,4] centrosymmetric 24 2×alternating gyroidal O 432 432 [4,3]+ enantiomorphic 24 symmetric hexoctahedral Oh m3m *432 [4,3] centrosymmetric 48 2×symmetric The crystal structures of biological molecules (such as protein structures) can only occur in the 11 enantiomorphic point groups, as biological molecules are invariably chiral. The protein assemblies themselves may have symmetries other than those given above, because they are not intrinsically restricted by the Crystallographic restriction theorem. For example the Rad52 DNA binding protein has an 11-fold rotational symmetry (in human), however, it must form crystals in one of the 11 enantiomorphic point groups given above.
Lattice systems
The distribution of the 14 Bravais lattice types into 7 lattice systems is given in the following table.
The 7 lattice systems The 14 Bravais Lattices triclinic (parallelepiped) 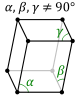
monoclinic (right prism with parallelogram base; here seen from above) simple base-centered 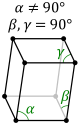
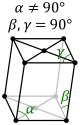
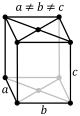
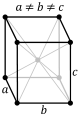
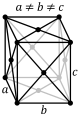
orthorhombic (cuboid) simple base-centered body-centered face-centered 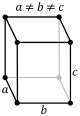
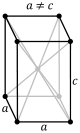
tetragonal (square cuboid) simple body-centered 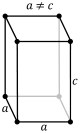
rhombohedral
(trigonal trapezohedron)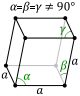
hexagonal (centered regular hexagon) 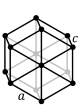
cubic
(isometric; cube)simple body-centered face-centered 

In geometry and crystallography, a Bravais lattice is a category of symmetry groups for translational symmetry in three directions, or correspondingly, a category of translation lattices.Such symmetry groups consist of translations by vectors of the form
where n1, n2, and n3 are integers and a1, a2, and a3 are three non-coplanar vectors, called primitive vectors.
These lattices are classified by space group of the translation lattice itself; there are 14 Bravais lattices in three dimensions; each can apply in one lattice system only. They represent the maximum symmetry a structure with the translational symmetry concerned can have.
All crystalline materials must, by definition fit in one of these arrangements (not including quasicrystals).
For convenience a Bravais lattice is depicted by a unit cell which is a factor 1, 2, 3 or 4 larger than the primitive cell. Depending on the symmetry of a crystal or other pattern, the fundamental domain is again smaller, up to a factor 48.
The Bravais lattices were studied by Moritz Ludwig Frankenheim (1801-1869), in 1842, who found that there were 15 Bravais lattices. This was corrected to 14 by A. Bravais in 1848.
See also
References
- Hahn, Theo, ed. (2002), International Tables for Crystallography, Volume A: Space Group Symmetry, A (5th ed.), Berlin, New York: Springer-Verlag, doi:10.1107/97809553602060000100, ISBN 978-0-7923-6590-7, http://it.iucr.org/A/
External links
- Overview of the 32 groups
- Mineral galleries - Symmetry
- all cubic crystal classes, forms and stereographic projections (interactive java applet)
- Crystal system at the Online Dictionary of Crystallography
- Crystal family at the Online Dictionary of Crystallography
- Lattice system at the Online Dictionary of Crystallography
- Conversion Primitive to Standard Conventional for VASP input files
- Learning Crystallography
Wikimedia Foundation. 2010.