- Stable isotope
-
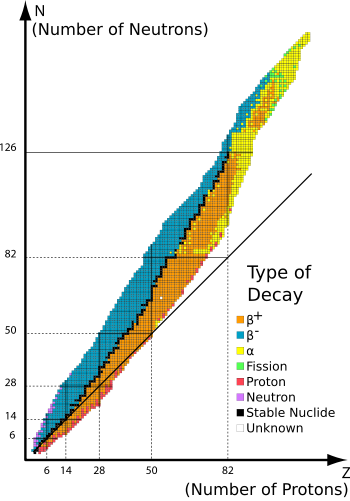 Graph of isotopes/nuclides by type of decay. Orange and blue nuclides are unstable, with the black squares between these regions representing stable nuclides. The unbroken line passing below many of the nuclides represents the theoretical position on the graph of nuclides for which proton number is the same as neutron number. The graph shows that elements with more than 20 protons must have more neutrons than protons, in order to be stable.
Graph of isotopes/nuclides by type of decay. Orange and blue nuclides are unstable, with the black squares between these regions representing stable nuclides. The unbroken line passing below many of the nuclides represents the theoretical position on the graph of nuclides for which proton number is the same as neutron number. The graph shows that elements with more than 20 protons must have more neutrons than protons, in order to be stable.
Stable isotopes are chemical isotopes that may or may not be radioactive, but if radioactive, have half-lives too long to be measured.
Only 90 nuclides from the first 40 elements are energetically stable to any kind of decay save proton decay, in theory (see list of nuclides). An additional 165 are theoretically unstable to known types of decay, but no evidence of decay has ever been observed, for a total of 255 nuclides for which there is no evidence of radioactivity. By this definition, there are 255 known stable nuclides of the 80 elements which have one or more stable isotopes. A list of these is given at the end of this article.
Of the 80 elements with one or more stable isotopes, twenty-six have only a single stable isotope, and are thus termed monoisotopic, and the rest have more than one stable isotope. One element (tin) has ten stable isotopes, the largest number known for an element.
Properties of stable isotopes
Different isotopes of the same element (whether stable or unstable) have nearly the same chemical characteristics and therefore behave almost identically in biology (a notable exception is the isotopes of hydrogen—see heavy water). The mass differences, due to a difference in the number of neutrons, will result in partial separation of the light isotopes from the heavy isotopes during chemical reactions and during physical processes such as diffusion and vaporization. This process is called isotope fractionation. For example, the difference in mass between the two stable isotopes of hydrogen, 1H (1 proton, no neutron, also known as protium) and 2H (1 proton, 1 neutron, also known as deuterium) is almost 100%. Therefore, a significant fractionation will occur.
Study of stable isotopes
Commonly analysed stable isotopes include oxygen, carbon, nitrogen, hydrogen and sulfur. These isotope systems have been under investigation for many years in order to study processes of isotope fractionation in natural systems because they are relatively simple to measure. Recent advances in mass spectrometry (i.e. multiple-collector inductively coupled plasma mass spectrometry) now enable the measurement of heavier stable isotopes, such as iron, copper, zinc, molybdenum, etc.
Stable isotopes have been used in botanical and plant biological investigations for many years, and more and more ecological and biological studies are finding stable isotopes (mostly carbon, nitrogen and oxygen) to be extremely useful. Other workers have used oxygen isotopes to reconstruct historical atmospheric temperatures, making them important tools for climate research. Measurements of ratios of one naturally occurring stable isotope to another play an important role in radiometric dating and isotope geochemistry, and also helpful for determining patterns of rainfall and movements of elements through living organisms, helping sort out food web dynamics in ecosystems.
Definition of stability, and natural isotopic presence
Most naturally occurring nuclides are stable (about 255; see list at the end of this article); and about 33 more (total of 288) are known radioactives with sufficiently long half-lives (also known) to occur "primordially." If the half-life of a nuclide is comparable to, or greater than, the Earth's age (4.5 billion years), a significant amount will have survived since the formation of the Solar System, and then is said to be primordial. It will then contribute in that way to the natural isotopic composition of a chemical element. Primordially present radioisotopes are easily detected with half-lives as short as 700 million years (e.g., 235U), although some primordial isotopes have been detected with half-lives as short as 80 million years (e.g., 244Pu). However, this is the present limit of detection, as the nuclide with the next-shortest half-life (niobium-92 with half-life 34.7 million years) has not been yet been detected in nature.
Many naturally-occurring radioisotopes (another 51 or so, for a total of about 339) exhibit still shorter half-lives than 80 million years, but they are made freshly, as daughter products of decay processes of primordial nuclides (for example, radium from uranium) or from ongoing energetic reactions, such as cosmogenic nuclides produced by present bombardment of Earth by cosmic rays (for example, carbon-14 made from nitrogen).
Many isotopes that are classed as stable (i.e. no radioactivity has been observed for them) are predicted to have extremely long half-lives (sometimes as high as 1018 years or more). If the predicted half-life falls into an experimentally accessible range, such isotopes have a chance to move from the list of stable nuclides to the radioactive category, once their activity is observed. Good examples are bismuth-209 and tungsten-180 which were formerly classed as stable, but have been recently (2003) found to be alpha-active. However, such nuclides do not change their status as primordial when they are found to be radioactive.
Most stable isotopes in the earth are believed to have been formed in processes of nucleosynthesis, either in the 'Big Bang', or in generations of stars that preceded the formation of the solar system. However, some stable isotopes also show abundance variations in the earth as a result of decay from long-lived radioactive nuclides. These decay-products are termed radiogenic isotopes, in order to distinguish them from the much larger group of 'non-radiogenic' isotopes.
Research areas
The so-called Island of Stability may reveal a number of long-lived or even stable atoms that are heavier (and with more protons) than lead.
Stable isotope fractionation
There are three types of isotope fractionation:
Isotopes per element
See also: List of elements by stability of isotopes, List of nuclides, and Beta-decay stable isobarsOf the known chemical elements, 80 elements have at least one stable nuclide. These comprise the first 82 elements from hydrogen to lead, with the exceptions of technetium (#43) and promethium (#61), which do not have any stable nuclides. As of December, 2010, there were a total of 255 known "stable" nuclides. In this definition, "stable" means a nuclide which has either never been observed to decay against the natural background. Thus, these elements have half-lives too long to be measured by any means, direct or indirect.
Only one element (tin) has 10 stable isotopes, and one (xenon) has nine stable isotopes. No elements have exactly eight stable isotopes, but four elements have seven stable isotopes, nine have six stable isotopes, nine have five stable isotopes, nine have four stable isotopes, five have three stable isotopes, 16 have two stable isotopes, and 26 have only a single stable isotope and are thus considered monoisotopic elements.[1] The mean number of stable isotopes for elements which have at least one such isotope, is 255/80 = 3.2.
"Magic numbers" and odd and even proton and neutron count
Stability of isotopes is affected by the ratio of protons to neutrons, and also by presence of certain "magic numbers" of neutrons or protons which represent closed and filled quantum shells. These quantum shells correspond to a set of energy levels within the shell model of the nucleus; filled shells, such as the filled shell of 50 protons for tin, confers unusual stability on the nuclide. As in the case of tin, a magic number for Z, the atomic number, tends to increase the number of stable isotopes for the element.
Just as in the case of electrons, which have the lowest energy state when they occur in pairs in a given orbital, nucleons (both protons and neutrons) exhibit a lower energy state when their number is even, rather than odd. This stability tends to prevent beta decay (in two steps) of many even-even nuclides into another even-even nuclide of the same mass number but lower energy (and of course with two more protons and two fewer neutrons), because decay proceeding one step at a time would have to pass through an odd-odd nuclide of higher energy. This makes for a larger number of stable even-even nuclides, up to three for some mass numbers, and up to seven for some atomic (proton) numbers. Conversely, of the 255 known stable nuclides, only four have both an odd number of protons and odd number of neutrons: hydrogen-2 (deuterium), lithium-6, boron-10 and nitrogen-14. Also, only four naturally occurring, radioactive odd-odd nuclides have a half-life over a billion years: potassium-40, vanadium-50, lanthanum-138 and tantalum-180m. Odd-odd primordial nuclides are rare because most odd-odd nuclei are highly unstable with respect to beta decay, because the decay products are even-even, and are therefore more strongly bound, due to nuclear pairing effects.[2]
Yet another effect of the instability of an odd number of either type of nucleons, is that odd-numbered elements tend to have fewer stable isotopes. Of the 26 monoisotopic elements that have only a single stable isotope, all but one have an odd atomic number — the single exception to both rules being beryllium. All of these elements also have an even number of neutrons, with the single exception again being beryllium.
Nuclear isomers, including a "stable" one
The count of 255 known stable nuclides includes Ta-180m, since even though its decay and instability is automatically implied by its notation of "metastable", still this has not yet been observed. All "stable" isotopes (stable by observation, not theory) are the ground states of nuclei, with the exception of tantalum-180m, which is the nuclear isomer or excited level (the ground state of this nucleus is radioactive with a very short half-life of 8 hours); but the decay of the excited nuclear isomer is extremely strongly forbidden by spin-parity selection rules. It has been reported experimentally by direct observation that the half-life of 180mTa to gamma decay must be more than 1015 years. Other possible modes of 180mTa decay (beta decay, electron capture and alpha decay) have also never been observed.
Primordial radioactive and naturally occurring non-primordial isotopes
Main articles: List of elements by stability of isotopes and primordial nuclideElements with more than 82 protons only have radioactive isotopes, although they can still occur naturally because their half-lives are more than about 2% of the time since the supernova nucleosynthesis of the elements from which our solar system was made. An extreme case of this is plutonium-244, which is still detectable from primordial reservoirs, even though it has a half-life of only 80 million years (1.8% of the solar system age). There exist about 33 naturally occurring radioactive primordial nuclides.
In about 50 known cases, elements with shorter half-lives than plutonium-244 are naturally observed on Earth, since as they are produced by cosmic rays (e.g., carbon-14), or else because (like radium and polonium) they occur in a decay chain of radioactive isotopes (primarily uranium and thorium), which have long-enough half-lives to be abundant primordially.
Still-unobserved decay
Main article: List of nuclidesIt is expected that continuous improvement of experimental sensitivity will allow discovery of very mild radioactivity (instability) of some isotopes that are considered stable today. For example, it wasn't until 2003 that bismuth-209 (the only naturally-occurring isotope of bismuth) was shown to be very mildly radioactive.[3] Many "stable" nuclides are possibly "meta-stable" in as much as they may be calculated to have an energy release[4] upon several possible kinds of radioactive decays.
Only 90 nuclides from the first 40 elements are theoretically stable to any sort of decay save proton decay (which has not been observed). The rest, starting with niobium-93, are theoretically unstable to spontaneous fission.
For processes other than spontaneous fission, other theoretical decay routes for heavier elements include:
- alpha decay - 70 heavy nuclides
- double beta decay (including double electron capture, electron-positron conversion and double positron decay) - 55 nuclides
- beta decay - Ta-180m
- electron capture - Te-123, Ta-180m
- isomeric transition - Ta-180m
- cluster decay and spontaneous fission - many heaviest nuclides
These include all nuclides of mass 201 and greater. Argon-36 is presently the lightest known "stable" nuclide which is theoretically unstable.
The positivity of energy release in these processes means that they are allowed kinematically (they do not violate the conservation of energy) and, thus, in principle, can occur. They are not observed due to strong but not absolute suppression, by spin-parity selection rules (for beta decays and isomeric transitions) or by the thickness of the potential barrier (for alpha and cluster decays and spontaneous fission).
Summary table for numbers of each class of nuclides
This is a summary table from List of nuclides. Note that numbers are not exact, and may change slightly in the future, as nuclides are observed to be radioactive, or new half-lives are determined to some precision. Note that only the 255 have any claim to stability, but that only 90 nuclides from the first 40 elements are theoretically stable to any process but proton decay.
Type of nuclide by stability class. Number of nuclides in class (exact number may change). Running total of nuclides in all classes to this point. Notes on running total. Theoretically stable to all but proton decay. 90 90 Includes first 40 elements. Proton decay yet to be observed. Energetically unstable to one or more known decay modes, but no decay yet seen. Considered stable until radioactivity confirmed. 165 255 Spontaneous fission possible for "stable" nuclides > niobium-93. Other mechanisms possible for heavier nuclides. Total is the classically stable nuclides Radioactive primordial nuclides. 33 288 Total primordials include Bi, U,Th, Pu, plus all stable nuclides. Radioactive nonprimordial, but naturally occurring on Earth. ~ 51 ~ 339 Cosmogenic nuclides from cosmic rays; daughters of radioactive primordials such as francium, etc. List of observationally-stable isotopes
In the list below, 90 nuclides have no predicted energetically-possible mode of decay, save proton decay. These are unmarked.
Other predicted (but not yet observed) modes of radioactive decay are noted as: A for alpha decay, B for beta decay, BB for double beta decay, E for electron capture, EE for double electron capture, and IT for isomeric transition. Because of the curve of binding energy, all nuclides from Z = 41 (niobium) and beyond, are theoretically unstable with regard to spontaneous fission SF (see list of nuclides for details), and many of the heavier nuclides are theoretically unstable to other processes as well.
- Hydrogen-1
- Hydrogen-2
- Helium-3
- Helium-4
- Lithium-6
- Lithium-7
- Beryllium-9
- Boron-10
- Boron-11
- Carbon-12
- Carbon-13
- Nitrogen-14
- Nitrogen-15
- Oxygen-16
- Oxygen-17
- Oxygen-18
- Fluorine-19
- Neon-20
- Neon-21
- Neon-22
- Sodium-23
- Magnesium-24
- Magnesium-25
- Magnesium-26
- Aluminium-27
- Silicon-28
- Silicon-29
- Silicon-30
- Phosphorus-31
- Sulfur-32
- Sulfur-33
- Sulfur-34
- Sulfur-36
- Chlorine-35
- Chlorine-37
- Argon-36 (EE)
- Argon-38
- Argon-40
- Potassium-39
- Potassium-41
- Calcium-40 (EE)
- Calcium-42
- Calcium-43
- Calcium-44
- Calcium-46 (BB)
- Scandium-45
- Titanium-46
- Titanium-47
- Titanium-48
- Titanium-49
- Titanium-50
- Vanadium-51
- Chromium-50 (EE)
- Chromium-52
- Chromium-53
- Chromium-54
- Manganese-55
- Iron-54 (EE)
- Iron-56
- Iron-57
- Iron-58
- Cobalt-59
- Nickel-58 (EE)
- Nickel-60
- Nickel-61
- Nickel-62
- Nickel-64
- Copper-63
- Copper-65
- Zinc-64 (EE)
- Zinc-66
- Zinc-67
- Zinc-68
- Zinc-70 (BB)
- Gallium-69
- Gallium-71
- Germanium-70
- Germanium-72
- Germanium-73
- Germanium-74
- Arsenic-75
- Selenium-74 (EE)
- Selenium-76
- Selenium-77
- Selenium-78
- Selenium-80 (BB)
- Bromine-79
- Bromine-81
- Krypton-78 (EE)
- Krypton-80
- Krypton-82
- Krypton-83
- Krypton-84
- Krypton-86 (BB)
- Rubidium-85
- Strontium-84 (EE)
- Strontium-86
- Strontium-87
- Strontium-88
- Yttrium-89
- Zirconium-90
- Zirconium-91
- Zirconium-92
- Zirconium-94 (BB)
- Niobium-93 (SF)
- Molybdenum-92 (EE)
- Molybdenum-94 (SF)
- Molybdenum-95 (SF)
- Molybdenum-96 (SF)
- Molybdenum-97 (SF)
- Molybdenum-98 (BB)
- Technetium - No stable isotopes
- Ruthenium-96 (EE)
- Ruthenium-98 (SF)
- Ruthenium-99 (SF)
- Ruthenium-100 (SF)
- Ruthenium-101 (SF)
- Ruthenium-102 (SF)
- Ruthenium-104 (BB)
- Rhodium-103 (SF)
- Palladium-102 (EE)
- Palladium-104 (SF)
- Palladium-105 (SF)
- Palladium-106 (SF)
- Palladium-108 (SF)
- Palladium-110 (BB)
- Silver-107 (SF)
- Silver-109 (SF)
- Cadmium-106 (EE)
- Cadmium-108 (EE)
- Cadmium-110 (SF)
- Cadmium-111 (SF)
- Cadmium-112 (SF)
- Cadmium-114 (BB)
- Indium-113 (SF)
- Tin-112 (EE)
- Tin-114 (SF)
- Tin-115 (SF)
- Tin-116 (SF)
- Tin-117 (SF)
- Tin-118 (SF)
- Tin-119 (SF)
- Tin-120 (SF)
- Tin-122 (BB)
- Tin-124 (BB)
- Antimony-121 (SF)
- Antimony-123 (SF)
- Tellurium-120 (EE)
- Tellurium-122 (SF)
- Tellurium-123 (E)
- Tellurium-124 (SF)
- Tellurium-125 (SF)
- Tellurium-126 (SF)
- Iodine-127 (SF)
- Xenon-124 (EE)
- Xenon-126 (EE)
- Xenon-128 (SF)
- Xenon-129 (SF)
- Xenon-130 (SF)
- Xenon-131 (SF)
- Xenon-132 (SF)
- Xenon-134 (BB)
- Xenon-136 (BB)
- Caesium-133 (SF)
- Barium-132 (EE)
- Barium-134 (SF)
- Barium-135 (SF)
- Barium-136 (SF)
- Barium-137 (SF)
- Barium-138 (SF)
- Lanthanum-139 (SF)
- Cerium-136 (EE)
- Cerium-138 (EE)
- Cerium-140 (SF)
- Cerium-142 (A, BB)
- Praseodymium-141 (SF)
- Neodymium-142 (SF)
- Neodymium-143 (A)
- Neodymium-145 (A)
- Neodymium-146 (A, BB)
- Neodymium-148 (A, BB)
- Promethium - No stable isotopes
- Samarium-144 (EE)
- Samarium-149 (A)
- Samarium-150 (A)
- Samarium-152 (A)
- Samarium-154 (BB)
- Europium-153 (A)
- Gadolinium-154 (A)
- Gadolinium-155 (A)
- Gadolinium-156 (SF)
- Gadolinium-157 (SF)
- Gadolinium-158 (SF)
- Gadolinium-160 (BB)
- Terbium-159 (SF)
- Dysprosium-156 (A, EE)
- Dysprosium-158 (A, EE)
- Dysprosium-160 (A)
- Dysprosium-161 (A)
- Dysprosium-162 (A)
- Dysprosium-163 (SF)
- Dysprosium-164 (SF)
- Holmium-165 (A)
- Erbium-162 (A, EE)
- Erbium-164 (A, EE)
- Erbium-166 (A)
- Erbium-167 (A)
- Erbium-168 (A)
- Erbium-170 (A, BB)
- Thulium-169 (A)
- Ytterbium-168 (A, EE)
- Ytterbium-170 (A)
- Ytterbium-171 (A)
- Ytterbium-172 (A)
- Ytterbium-173 (A)
- Ytterbium-174 (A)
- Ytterbium-176 (A, BB)
- Lutetium-175 (A)
- Hafnium-176 (A)
- Hafnium-177 (A)
- Hafnium-178 (A)
- Hafnium-179 (A)
- Hafnium-180 (A)
- Tantalum-180m (A, B, E, IT)
- Tantalum-181 (A)
- Tungsten-182 (A)
- Tungsten-183 (A)
- Tungsten-184 (A)
- Tungsten-186 (A, BB)
- Rhenium-185 (A)
- Osmium-184 (A, EE)
- Osmium-187 (A)
- Osmium-188 (A)
- Osmium-189 (A)
- Osmium-190 (A)
- Osmium-192 (A, BB)
- Iridium-191 (A)
- Iridium-193 (A)
- Platinum-192 (A)
- Platinum-194 (A)
- Platinum-195 (A)
- Platinum-196 (A)
- Platinum-198 (A, BB)
- Gold-197 (A)
- Mercury-196 (A, EE)
- Mercury-198 (A)
- Mercury-199 (A)
- Mercury-200 (A)
- Mercury-201 (A)
- Mercury-202 (A)
- Mercury-204 (BB)
- Thallium-203 (A)
- Thallium-205 (A)
- Lead-204 (A)
- Lead-206 (A)
- Lead-207 (A)
- Lead-208 (A)
Abbreviations:
A for alpha decay, B for beta decay, BB for double beta decay, E for electron capture, EE for double electron capture, IT for isomeric transition.See also
- Table of nuclides
- List of nuclides (905 nuclides in order of stability, all with half-lives > one hour)
- Isotope geochemistry
- Radionuclide
- Mononuclidic element
- Primordial nuclide
- List of elements by stability of isotopes
References
- ^ Sonzogni, Alejandro. "Interactive Chart of Nuclides". National Nuclear Data Center: Brook haven National Laboratory. http://www.nndc.bnl.gov/chart/. Retrieved 2008-06-06.
- ^ Various (2002). Lide, David R.. ed. Handbook of Chemistry & Physics (88th ed.). CRC. ISBN 0-8493-0486-5. OCLC 179976746. http://www.hbcpnetbase.com/. Retrieved 2008-05-23.
- ^ "WWW Table of Radioactive Isotopes". http://nucleardata.nuclear.lu.se/nucleardata/toi/listnuc.asp?sql=&HlifeMin=1e30&tMinStr=1e30+s&HlifeMax=1e40&tMaxStr=1e+40+s.
- ^ AME2003 Atomic Mass Evaluation from the National Nuclear Data Center
Book references
- Various (2002). Lide, David R.. ed. Handbook of Chemistry & Physics (88th ed.). CRC. ISBN 0-8493-0486-5. OCLC 179976746. http://www.hbcpnetbase.com/. Retrieved 2008-05-23.
External links
AlphaDelta: Stable Isotope fractionation calculator - http://www2.ggl.ulaval.ca/cgi-bin/isotope/generisotope.cgi
- National Isotope Development Center Reference information on isotopes, and coordination and management of isotope production, availability, and distribution
- Isotope Development & Production for Research and Applications (IDPRA) U.S. Department of Energy program for isotope production and production research and development
Categories:- Isotopes
Wikimedia Foundation. 2010.

