- Nuclear isomer
-
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons (protons or neutrons). "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states (which ordinarily last on the order of 10−12 seconds). As a result, the term "metastable" is usually restricted to refer to isomers with half-lives of 10−9 seconds or longer.
Occasionally the half-lives are far longer than this, and can last minutes, hours, or (in one known case 180m
73Ta) at least 1015 years. Sometimes, the gamma decay from a metastable state is given the special name of an isomeric transition, but save for the long-lived nature of the meta-stable parent nuclear isomer, this process resembles shorter-lived gamma decays in all other aspects.The first nuclear isomers (Uranium Z/Uranium X2, now known as 234
91Pa/234m
91Pa) were discovered by Otto Hahn in 1921.[1]Contents
Nucleus
The nucleus of a nuclear isomer occupies a higher energy state than the corresponding non-excited nucleus, which exists in the lowest energy state, called the ground state. In an excited state, one or more of the protons or neutrons in a nucleus occupy a nuclear orbital of higher energy than an available nuclear orbital of lower energy. These states are analogous to excited states of electrons in atoms.
Excited atomic states decay by fluorescence which usually involves emission of light near the visible range. However, because of the much higher binding energies involved in nuclear processes, most nuclear excited states decay instead by gamma ray emission. For example, a well-known nuclear isomer used in medical procedure is technetium-99m, which decays with a half-life of about 6 hours, by emitting a gamma ray of 140 kiloelectron-volts of energy (this is close to the energy of medical diagnostic X-rays).
Internal conversion
Metastable isomers may also decay by internal conversion — a process in which the energy of nuclear de-excitation is not emitted as a gamma ray, but instead used to accelerate one of the inner electrons of the atom, so that it leaves at high speed and energy. This process is only possible because inner atomic electrons penetrate the nucleus, where they are subject to the intense electric fields which result when the protons of the nucleus re-arrange in a different way. In nuclei which are far from stability in energy, still other decay modes are known.
Metastable isomers
Metastable isomers can be produced through nuclear fusion or other nuclear reactions. A nucleus thus produced generally starts its existence in an excited state that relaxes through the emission of one or more gamma rays or conversion electrons. However, sometimes it happens that the de-excitation does not proceed rapidly all the way to the nuclear ground state. This usually occurs because of the formation of an intermediate excited state with a spin far different from that of the ground state. Gamma-ray emission is far slower (is "hindered") if the spin of the post-emission state is very different from that of the emitting state, particularly if the excitation energy is low. The excited state in this situation is therefore a good candidate to be metastable if there are no other states of intermediate spin with excitation energies less than that of the metastable state.
Metastable isomers of a particular isotope are usually designated with an "m" (or, in the case of isotopes with more than one isomer, m2, m3, and so on). This designation is placed after the mass number of the atom; for example, Cobalt-58m (abbreviated 58m
27Co, where 27 is the atomic number of cobalt). Increasing indices, m, m2, etc., correlate with increasing levels of excitation energy stored in each of the isomeric states (e.g., hafnium-177m2 or 177m2
72Hf).A different kind of metastable nuclear state (isomer) is the fission isomer or shape isomer. Most actinoid nuclei, in their ground states, are not spherical, but rather spheroidal—specifically, prolate, with an axis of symmetry longer than the other axes (similar to an American football or rugby ball, although with a less pronounced departure from spherical symmetry).[citation needed] In some of these, quantum-mechanical states can exist in which the distribution of protons and neutrons is farther yet from spherical (in fact, about as non-spherical as an American football), so much so that de-excitation to the nuclear ground state is strongly hindered. In general, these states either de-excite to the ground state (albeit far more slowly than a "usual" excited state) or undergo spontaneous fission with half-lives of the order of nanoseconds or microseconds—a very short time, but many orders of magnitude longer than the half-life of a more usual nuclear excited state. Fission isomers are usually denoted with a postscript or superscript "f" rather than "m", so that a fission isomer in, e.g., plutonium 240 is denoted plutonium-240f or 240f
94Pu.Nearly-stable isomers
Most nuclear excited states are very unstable, and radiate away the extra energy immediately (on the order of 10−12 seconds). As a result, the term is usually restricted to refer to isomers with half-lives of 10−9 seconds or longer. Quantum mechanics predicts that certain atomic species will possess isomers with unusually long lifetimes even by this stricter standard, and so have interesting properties. By definition, there is no such thing as a "stable" isomer; however, some are so long-lived as to be nearly stable, and can be produced and observed in quantity.
The most stable nuclear isomer occurring in nature is 180m
73Ta, which is present in all tantalum samples at about 1 part in 8,300. Its half-life is at least 1015 years, markedly longer than the age of the universe. This remarkable persistence results from the fact that the excitation energy of the isomeric state is low, and both gamma de-excitation to the 180
Ta ground state (which itself is radioactive by beta decay, with a half-life of only 8 hours), and also direct beta decay to hafnium or tungsten are all suppressed, owing to spin mismatches. The origin of this isomer is mysterious, though it is believed to have been formed in supernovae (as are most other heavy elements). When it relaxes to its ground state, it releases a photon with an energy of 75 keV.It was first reported in 1988 by Collins[2] that 180m
Ta can be forced to release its energy by weaker x-rays. After 11 years of controversy those claims were confirmed in 1999 by Belic and co-workers in the Stuttgart nuclear physics group.[3]Another reasonably stable nuclear isomer (with a half-life of 31 years) is 178m2
72Hf, which has the highest excitation energy of any comparably long-lived isomer. One gram of pure 178m2
Hf contains approximately 1.33 gigajoules of energy, the equivalent of exploding about 315 kg (690 lb) of TNT. Further, in the natural decay of 178m2
Hf, the energy is released as gamma rays with a total energy of 2.45 MeV. As with 180m
Ta, there are disputed reports that 178m2
Hf can be stimulated into releasing its energy, and as a result the substance is being studied as a possible source for gamma ray lasers. These reports also indicate that the energy is released very quickly, so that 178m2
Hf can produce extremely high powers (on the order of exawatts). Other isomers have also been investigated as possible media for gamma-ray stimulated emission.[4][5]Holmium has an interesting[why?] nuclear isomer, 166m1
67Ho with a half-life of 1,200 years, which is nearly the longest half-life of any holmium radionuclide (only 163
Ho, with a half-life of 4,570 years is longer).229
90Th has a remarkably low-lying metastable isomer, only 7.6 ± 0.5 electron volts above the ground state, as calculated from spectroscopic measurements. This direct decay has not been observed, however. If this isomer were to decay it would produce a gamma ray (defined by its origin, not its wavelength) in the ultraviolet range. These "ultraviolet gamma rays" were thought to have been detected at one time,[6] but this observation has since been found to be from nitrogen gas excited by higher energy emissions.[7]Applications
Hafnium[8][9] and tantalum[citation needed] isomers have been considered in some quarters as weapons that could be used to circumvent the Nuclear Non-Proliferation Treaty, since they can be induced to emit very strong gamma radiation. DARPA has (or had) a program to investigate this use of both nuclear isomers.[10] The potential to trigger an abrupt release of energy from nuclear isotopes, a prerequisite to their use in such weapons, is disputed. Nonetheless a 12-member Hafnium Isomer Production Panel (HIPP) was created to assess means of mass producing the isotope.[11]
Technetium isomers 99m
43Tc (with a half-life of 6.01 hours) and 95m
43Tc (with a half-life of 61 days) are used in medical and industrial applications.Nuclear batteries
Nuclear batteries in development use small amounts (milligrams and microcuries) of radioisotopes with high energy densities. In one design, radioactive material sits atop a device with adjacent layers of P-type and N-type silicon, so that ionizing radiation directly penetrates the junction and creates electron-hole pairs. Nuclear isomers could replace other isotopes, and with further development it may be possible to turn them on and off as needed. Current candidates for such use include 108Ag, 166Ho, 177Lu, and 241Am. As of 2004 the only isomer which had been successfully triggered was 180Ta, which required more photon energy to trigger than was released.[12]
Fission of an isotope such as 177Lu releases gamma rays by decay through a series of internal energy levels within the nucleus, and it is thought that by learning the triggering cross sections with sufficient accuracy, it may be possible to create energy stores that are 106 times more concentrated than high explosive or other traditional chemical energy storage.[12]
Decay processes
Isomers decay to lower energy states of the nuclide through two isomeric transitions:
- γ (gamma) emission (emission of a high-energy photon)
- internal conversion (the energy is used to ionize the atom)
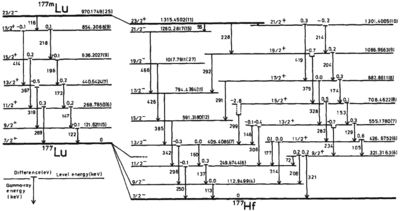
Isomers may also decay into other elements, though the rate of decay may differ between isomers. For example, 177mLu beta decays to 177Hf with half-life 160.4 d, or can undergo internal transition to 177Lu with half-life 160.4 d, then beta decays to 177Hf with half-life 6.68 d.[12]
See also
- Induced gamma emission
- Isomeric shift
References
- ^ Hahn, Otto (1921). "Über ein neues radioaktives Zerfallsprodukt im Uran". Die Naturwissenschaften 9 (5): 84. Bibcode 1921NW......9...84H. doi:10.1007/BF01491321.
- ^ C.B. Collins et al. (1988). "Depopulation of the isomeric state 180Tam by the reaction 180Tam(γ,γ′)180Ta". Phys. Rev. C 37: 2267–2269. Bibcode 1988PhRvC..37.2267C. doi:10.1103/PhysRevC.37.2267. http://www.hafniumisomer.org/isomer/180ta.pdf.
- ^ D. Belic et al. (1999). "Photoactivation of 180Tam and Its Implications for the Nucleosynthesis of Nature's Rarest Naturally Occurring Isotope". Phys. Rev. Lett. 83 (25): 5242. Bibcode 1999PhRvL..83.5242B. doi:10.1103/PhysRevLett.83.5242.
- ^ "UNH researchers search for stimulated gamma ray emission". UNH Nuclear Physics Group. 1997. Archived from the original on 5 September 2006. http://web.archive.org/web/20060905160103/http://einstein.unh.edu/nuclear/NucNews/graser_news.html. Retrieved 1 June 2006.
- ^ P. M. Walker and J. J. Carroll (2007). "Nuclear Isomers: Recipes from the Past and Ingredients for the Future". Nuclear Physics News 17 (2): 11. doi:10.1080/10506890701404206. http://epubs.surrey.ac.uk/cgi/viewcontent.cgi?article=1060&context=physicspapers.
- ^ R.W. Shaw, J.P. Young, S.P. Cooper, O.F. Webb (1999-02-08). "Spontaneous Ultraviolet Emission from 233Uranium/229Thorium Samples". Physical Review Letters 82 (6): 1109–1111. Bibcode 1999PhRvL..82.1109S. doi:10.1103/PhysRevLett.82.1109.
- ^ S.B. Utter et al. (1999). "Reexamination of the Optical Gamma Ray Decay in 229Th". Phys. Rev. Lett. 82 (3): 505–508. Bibcode 1999PhRvL..82..505U. doi:10.1103/PhysRevLett.82.505.
- ^ David Hambling (16 August 2003). "Gamma-ray weapons". Reuters EurekAlert. New Scientist. http://www.eurekalert.org/pub_releases/2003-08/ns-gw081303.php. Retrieved 12 December 2010.
- ^ Jeff Hecht (19 June 2006). "A perverse military strategy". New Scientist. http://www.newscientist.com/article/mg19025562.200-a-perverse-military-strategy.html. Retrieved 12 December 2010.
- ^ S. Weinberger (28 March 2004). "Scary things come in small packages". Sunday Supplement Magazine. Washington Post. http://www.washingtonpost.com/ac2/wp-dyn?pagename=article&contentId=A22099-2004Mar24¬Found=true. Retrieved 2009-05-03.
- ^ "Superbomb ignites science dispute". San Francisco Chronicle. 2003-09-28. http://www.commondreams.org/headlines03/0928-07.htm.
- ^ a b c M.S. Litz and G. Merkel (2004-12-00 [sic]). "Controlled extraction of energy from nuclear isomers". http://www.dtic.mil/cgi-bin/GetTRDoc?Location=U2&doc=GetTRDoc.pdf&AD=ADA433348.
External links
- Research group which presented initial claims of hafnium nuclear isomer de-excitation control. – The Center for Quantum Electronics, The University of Texas at Dallas.
- JASON Defense Advisory Group report on high energy nuclear materials mentioned in the Washington Post story above
- Bertram Schwarzschild (May 2004). "Conflicting Results on a Long-Lived Nuclear Isomer of Hafnium Have Wider Implications". Physics Today: 21. http://scitation.aip.org/journals/doc/PHTOAD-ft/vol_57/iss_5/21_1.shtml. login required?
- Confidence for Hafnium Isomer Triggering in 2006. – The Center for Quantum Electronics, The University of Texas at Dallas.
- Reprints of articles about nuclear isomers in peer reviewed journals. – The Center for Quantum Electronics, The University of Texas at Dallas.
Categories:
Wikimedia Foundation. 2010.