- Cobalt(II) bromide
-
Cobalt(II) bromide 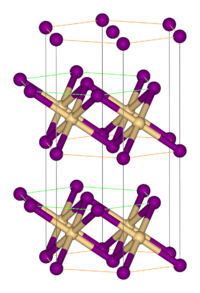
Identifiers CAS number 7789-43-7 
PubChem 24610 ChemSpider 23012 
RTECS number GF9595000 Jmol-3D images Image 1 - [Co](Br)Br
Properties Molecular formula CoBr2, CoBr2.6H2O, CoBr2.2H2O Molar mass 218.74 g/mol Appearance Green powder Density 4.909 g/cm3 Melting point 678 °C
Solubility in water 112 g/100 mL Structure Crystal structure Rhombohedral, hP3, SpaceGroup = P-3m1, No. 164 Coordination
geometryoctahedral Hazards MSDS Fisher Scientific EU Index Not listed R-phrases R36, R37, R38 S-phrases S26, S37, S39, S45, S28A NFPA 704 Flash point Non-flammable LD50 406 mg/kg Related compounds Other anions cobalt(II) fluoride
cobalt(II) chloride
cobalt(II) iodideOther cations iron(II) bromide
nickel(II) bromide bromide (verify) (what is:
bromide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cobalt(II) bromide (CoBr2) is an inorganic compound used primarily as a catalyst in some processes.
Contents
Properties
When anhydrous, cobalt(II) bromide appears as green crystals. The hexahydrate loses four waters of crystallization molecules at 100°C forming the dihydrate:
- CoBr2.6H2O → CoBr2.2H2O + 4H2O
Further heating to 130°C Produces the anhydrous form:
- CoBr2.4H2O → CoBr2
The anhydrous form will then melts at 678°C.[1][2] At higher temperatures, cobalt(II) bromide reacts with oxygen, forming cobalt(II,III) oxide and bromine vapor.
Preparation
Cobalt(II) bromide can be prepared as a hydrate by the reaction of cobalt hydroxide with hydrobromic acid:
- Co(OH)2(s) + 2HBr(aq) → CoBr2.6H2O(aq)
Anhydrous cobalt(II) bromide may be prepared through the direct reaction of elemental cobalt and liquid bromine.[3][4][5]
Uses
Cobalt(II) bromide may be used as a catalyst in the oxidation of some organic compounds with cobalt(III).[6]
Health issues
Exposure to large amounts of cobalt(II) can cause cobalt poisoning.[7] Bromide is also mildly toxic.
References
- ^ American Elements: Cobalt Bromide Supplier & Tech Info
- ^ WebElements Periodic Table of the Elements
- ^ WebElements Periodic Table of the Elements | Cobalt | Essential information
- ^ Chemical Properties and Reaction Tendencies
- ^ Pilgaard Solutions: Cobalt
- ^ Energy Citations Database (ECD) - - Document #7222610
- ^ http://www.chrismanual.com/C/COB.pdf
Cobalt compounds Categories:- Cobalt compounds
- Bromides
- Inorganic compound stubs
Wikimedia Foundation. 2010.

