- Flumazenil
-
Flumazenil 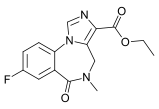
Systematic (IUPAC) name ethyl 12-fluoro- 8-methyl- 9-oxo- 2,4,8- triazatricyclo [8.4.0.02,6] tetradeca- 1(10),3,5,11,13- pentaene- 5-carboxylate Clinical data Trade names Romazicon AHFS/Drugs.com monograph Pregnancy cat. B3(AU) C Legal status ? Routes Intravenous Pharmacokinetic data Metabolism Hepatic Half-life 7-15 min (initial)
20-30 min (brain)
40-80 min (terminal)Excretion Urine 90-95%
Feces 5-10%Identifiers CAS number 78755-81-4 ATC code V03AB25 PubChem CID 3373 DrugBank APRD00974 ChemSpider 3256 
UNII 40P7XK9392 
KEGG D00697 
ChEBI CHEBI:5103 
ChEMBL CHEMBL407 
Synonyms ethyl 8-fluoro- 5,6-dihydro- 5-methyl- 6-oxo- 4H- imidazo [1,5-a] [1,4] benzodiazepine- 3-carboxylate Chemical data Formula C15H14FN3O3 Mol. mass 303.288 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Flumazenil (also known as flumazepil, code name Ro 15-1788, trade names Anexate, Lanexat, Mazicon, Romazicon) is a benzodiazepine antagonist available for injection only, and the only benzodiazepine receptor antagonist on the market today.
It was first introduced in 1987 by Hoffmann-La Roche under the trade name Anexate, but only approved by the FDA on December 20, 1991. Some years ago an oral preparation was under development [1], though it had low bio-availability and was thus abandoned. There is hope that oral flumazenil or other benzodiazepine antagonists such as B-carbolines will be developed in the future.
Contents
Uses
Flumazenil is of benefit in patients who become excessively drowsy after benzodiazepines are used for either diagnostic or therapeutic procedures.[2]
It has been used as an antidote in the treatment of benzodiazepine overdoses.[2] It reverses the effects of benzodiazepines by competitive inhibition at the benzodiazepine binding site on the GABAA receptor. There are many complications that must be taken into consideration when used in the acute care setting.[2]
It has been found to be effective in overdoses of non-benzodiazepine sleep enhancers, namely zolpidem and zaleplon.[3]
There is some evidence that flumazenil is effective for protracted benzodiazepine withdrawal (or benzodiazepine post-withdrawal syndrome) with promising results [4][5][6], though larger studies with more regular dosing intervals are needed to confirm this. There have also been some studies investigating the drugs effect in reducing benzodiazapine tolerance [7][8], with promising results. In binding to the benzodiazepine receptor, flumazenil may reset the receptors sensitivity, restoring the receptor to its pre-drug affinity. [9]
It has also been used in hepatic encephalopathy, though results have been mixed.[10][11]
The onset of action is rapid and usually effects are seen within one to two minutes. The peak effect is seen at six to ten minutes. The recommended dose for adults is 200 μg every 1–2 minutes until the effect is seen, to a maximum of 3 mg per hour. It is available as a clear, colourless solution for intravenous injection, containing 500 μg in 5 mL.
Many benzodiazepines (including midazolam) have longer half-lives than flumazenil. Therefore, repeat doses of flumazenil may be required to prevent recurrent symptoms of overdosage once the initial dose of flumazenil wears off. It is hepatically metabolised to inactive compounds which are excreted in the urine. Subjects who are physically dependent on benzodiazepines may suffer benzodiazepine withdrawal symptoms, including seizure, upon administration of flumazenil.
It is not recommended for routine use in those with a decreased level of consciousness.[12]
PET radioligand
Radiolabeled with the radioactive isotope carbon-11 flumazenil may be used as a radioligand in neuroimaging with positron emission tomography to visualize the distribution of GABAA receptors in the human brain.[13]
Clinical pharmacology
Flumazenil, an imidazobenzodiazepine derivative, antagonizes the actions of benzodiazepines on the central nervous system. Flumazenil competitively inhibits the activity at the benzodiazepine recognition site on the GABA / benzodiazepine receptor complex. Flumazenil is a weak partial agonist in some animal models of activity, but has little or no agonist activity in humans.
Flumazenil does not antagonize all of the central nervous system effects of drugs affecting GABA-ergic neurons by means other than the benzodiazepine receptor (including ethanol, barbiturates, or general anesthetics) and does not reverse the effects of opioids.
In animals pretreated with high doses of benzodiazepines over several weeks, Flumazenil injection elicited symptoms of benzodiazepine withdrawal, including seizures. A similar effect was seen in adult human subjects.
Pharmacodynamics
Intravenous Flumazenil has been shown to antagonize sedation, impairment of recall, psychomotor impairment and ventilatory depression produced by benzodiazepines in healthy human volunteers.
The duration and degree of reversal of sedative benzodiazepine effects are related to the dose and plasma concentrations of Flumazenil.
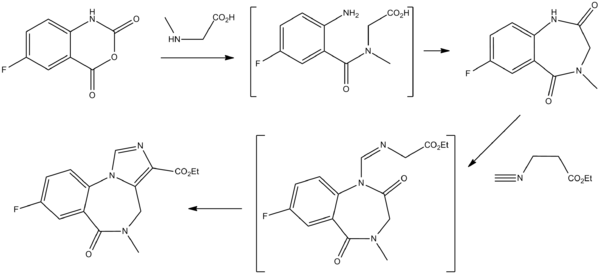
Chemistry
Gerecke, M.; Hunkeler, W.; Kyburz, E.; Mohler, H.; Pieri, L.; Pole, P.; 1982, U.S. Patent 4,316,839.
See also
- Benzodiazepine overdose
- Benzodiazepine
- Bretazenil
- Imidazenil
References
- ^ Higgitt, Lader and Fonagy, 1986
- ^ a b c Goldfrank, Lewis R. (2002). Goldfrank's toxicologic emergencies. New York: McGraw-Hill Medical Publ. Division. ISBN 0-07-136001-8. http://books.google.com/?id=HVYyRsuUEc0C&pg=PA948&dq=flumazenil+not+to+be+used+in+overdose.
- ^ Nelson, Lewis H.; Flomenbaum, Neal; Goldfrank, Lewis R.; Hoffman, Robert Louis; Howland, Mary Deems; Neal A. Lewin (2006). Goldfrank's toxicologic emergencies. New York: McGraw-Hill, Medical Pub. Division. ISBN 0-07-147914-7.
- ^ Professor Malcolm Lader (1992). "A pilot study of the effects of flumazenil on symptoms persisting after benzodiazepine withdrawal". Journal of Psychopharmacology. http://www.bcnc.org.uk/flumazenil.html.
- ^ Lader MB, Morton SV. A pilot study of the effects of flumazenil on symptoms persisting after benzodiazepine withdrawal. JPsychopharmacol. 1992; 6: 19-28.
- ^ Saxon L, Hjemdahl P, Hiltunen AJ, Borg S (May 1997). "Effects of flumazenil in the treatment of benzodiazepine withdrawal--a double-blind pilot study" (PDF). Psychopharmacology (Berl.) 131 (2): 153–60. doi:10.1007/s002130050278. PMID 9201803. http://www.springerlink.com/content/2vpf562teffglej5/fulltext.pdf
- ^ Savic I, Widen L, Stone-Elander S. Feasibility of reversing benzodiazepine tolerance with flumazenil. Lancet 1991; 337: 133-137.
- ^ Savic, Widen and Stone-Elander, 1991)
- ^ Nutt and Costello, 1988
- ^ Goulenok C, Bernard B, Cadranel JF, et al. (March 2002). "Flumazenil vs. placebo in hepatic encephalopathy in patients with cirrhosis: a meta-analysis". Aliment. Pharmacol. Ther. 16 (3): 361–72. doi:10.1046/j.1365-2036.2002.01191.x. PMID 11876688. http://www3.interscience.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=0269-2813&date=2002&volume=16&issue=3&spage=361.
- ^ Als-Nielsen B, Gluud LL, Gluud C (2004). Als-Nielsen, Bodil. ed. "Benzodiazepine receptor antagonists for hepatic encephalopathy". Cochrane Database Syst Rev (2): CD002798. doi:10.1002/14651858.CD002798.pub2. PMID 15106178.
- ^ Wood, Lawrence D. H.; Hall, Jesse B.; Schmidt, Gregory D. 1952 (2005). Principles of critical care. McGraw-Hill Professional. ISBN 0-07-141640-4. http://books.google.com/?id=Ss7gzGwk78gC&pg=RA11-PA1505&lpg=RA11-PA1505&dq=flumazenil+not+recommended+in+overdose.
- ^ Alexander Hammers, Matthias J. Koepp, Mark P. Richardson, Rene Hurlemann, David J. Brooks & John S. Duncan (June 2003). "Grey and white matter flumazenil binding in neocortical epilepsy with normal MRI. A PET study of 44 patients". Brain 126 (Pt 6): 1300–1308. doi:10.1093/brain/awg138. PMID 12764053. http://brain.oxfordjournals.org/cgi/content/abstract/126/6/1300.
Other
- Romazicon product information, Roche USA
External links
- Flumazenil drug label/data at Daily Med from U.S. National Library of Medicine, National Institutes of Health.
Antidotes (V03AB) Nervous system Barbiturate overdoseBemegride • EthamivanBenzodiazepine overdoseCyprodenate • FlumazenilGHB overdoseCardiovascular Other Paracetamol toxicity (Acetaminophen)OtherPrednisolone/promethazine • oxidizing agent (potassium permanganate) • iodine-131 (Potassium iodide) • Methylthioninium chloride#Emetic GABAergics Receptor
ligandsAgonists: Main site: Bamaluzole • Gaboxadol • Ibotenic acid • Isoguvacine • Isonipecotic acid • Muscimol (Amanita Muscaria) • Progabide • SL 75102 • Thiomuscimol • Tolgabide; Positive allosteric modulators: Barbiturates • Benzodiazepines • Carbamates • Chlormezanone • Clomethiazole • Ethanol (Alcohol) • Etomidate • Kavalactones (Kava) • Loreclezole • Metomidate • Neuroactive steroids • Nonbenzodiazepines (β-Carbolines, Cyclopyrrolones, Imidazopyridines, Pyrazolopyrimidines, etc.) • Phenols • Piperidinediones • Propanidid • Pyrazolopyridines • Quinazolinones • ROD-188 • Skullcap • Stiripentol • Valerenic acid (Valerian)
Antagonists: Main site: Bicuculline • Gabazine • Pitrazepin; Negative allosteric modulators: α5IA • Bilobalide • Cicutoxin • Cyclothiazide • DMCM • Flumazenil • Flurothyl • Furosemide • L-655,708 • Oenanthotoxin • Penicillin • Pentylenetetrazol • Picrotoxin • PWZ-029 • Ro15-4513 • Sarmazenil • Suritozole • Thujone (Absinthe) • Thiocolchicoside • ZK-93426
* See Template:GABAAergics for a full list of GABAA positive allosteric modulators.Agonists: Main site: CACA • CAMP • GABOB • N(4)-chloroacetylcytosine arabinoside • Progabide • Tolgabide
Antagonists: Main site: Bilobalide • TPMPAReuptake
inhibitorsPlasmalemmalGAT inhibitorsCI-966 • Deramciclane • EF-1502 • Gabaculine • Guvacine • Nipecotic acid • NNC 05-2090 • SKF-89976A • SNAP-5114 • TiagabineEnzyme
inhibitorsGAD inhibitorsAllylglycineGABA-T inhibitors3-Hydrazinopropionic acid • Aminooxyacetic acid • Gabaculine • Isoniazid • Phenelzine • Phenylethylidenehydrazine • Sodium valproate • Valnoctamide • Valproate pivoxil • Valproate semisodium (Divalproex sodium) • Valproic acid • Valpromide • VigabatrinOthers Glutamate • GlutamineOthersCategories:- Antidotes
- Imidazobenzodiazepines
- Organofluorides
- GABA antagonists
- Convulsants
- Anxiogenics
- Carboxylate esters
- Lactams
- Hoffmann-La Roche
Wikimedia Foundation. 2010.