- Stiripentol
-
Stiripentol 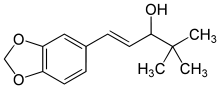
Systematic (IUPAC) name (RS)-(E)-4,4-dimethyl-1-[3,4(methylenedioxy)-phenyl]-1-penten-3-ol Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status ? Routes Oral Identifiers CAS number 49763-96-4 
ATC code N03AX17 PubChem CID 5311454 ChemSpider 4470940 
UNII R02XOT8V8I 
KEGG D05928 
Chemical data Formula C14H18O3 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Stiripentol (marketed as Diacomit by Laboratoires Biocodex) is an anticonvulsant drug used in the treatment of epilepsy. It is unrelated to other anticonvulsants and belongs to the group of aromatic allylic alcohols.
Contents
Mechanism of action
As with most anticonvulsants, the precise mechanism is unknown. It has been shown to have anticonvulsant effects on its own.
It increases GABA transmission. At clinically relevant concentrations, STP enhances central GABA transmission through a barbiturate-like effect, since it increases the duration of opening of GABA-A receptors channels in hippocampal slices.[1] It has also been shown that STP may increase the GABA levels in brain tissues and by interfering with its uptake and its metabolism.[2]
It also improves the effectiveness of many other anticonvulsants, possibly due to it inhibiting certain enzymes. This slows the drug's metabolism, increasing blood plasma levels.
History
In December 2001 the European Medicines Agency (EMA) granted stiripentol orphan drug status (designation number EU/3/01/071) for the treatment of severe myoclonic epilepsy in infancy (SMEI, also known as Dravet's syndrome). On 4 January 2007, the EMA granted the drug a marketing authorisation that is valid throughout the European Union.
Indications and usage
It is indicated as an adjunctive therapy with sodium valproate and clobazam for treating severe myoclonic epilepsy in infancy (SMEI)[3] whose seizures are not adequately controlled with clobazam and valproate. Children with SMEI develop often intractable seizures during their first year of life and mental retardation follows. Small-scale trials have show remarkably high response rates, with a significant minority of those treated becoming seizure free.
In addition, it may be used to treat refractory childhood epilepsy in conjunction with carbamazepine. It appears to be less effective in adolescents and adults.
Dosing
Stiripentol is available as a gelatine capsule (250 mg, 500 mg) and as a sachet of powder to make a drinkable suspension (250 mg, 500 mg).
Initial dose is 50 mg/kg per day. This may be increased up to 100 mg/kg per day, with a maximum of 4g. The dose to be divided into two or three with meals. The does of other anticonvulsants may have to be reduced (possibly up to 50%).
Side effects
Side effects are largely due to the increase in plasma concentrations of other anticonvulsants and can be reduced by lowering the dose of those drugs. Nausea and vomiting are particularly noted when used in combination with sodium valproate.
Drug interactions
Stiripentol inhibits several cytochrome P450 isoenzymes and so interacts with many anticonvulsants and other medicines. This is both a strength and weakness. It appears to increase the potency of phenobarbital, primidone, phenytoin, carbamazepine, clobazam and diazepam. For example, blood levels of carbamazepine can be maintained while reducing the dose by 50%.
References
- ^ Quilichini PP, Chiron C, Ben-Ari Y, Gozlan H (2006). "Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABA-A receptor channels". Epilepsia : PR 47 (4): 704–16. doi:10.1111/j.1528-1167.2006.00497.x. PMID 16650136. http://www3.interscience.wiley.com/cgi-bin/fulltext/118726053/PDFSTART.
- ^ Trojnar MK, Wojtal K, Trojnar MP, Czuczwar SJ (2005). "Stiripentol. A novel antiepileptic drug". Pharmacological reports : PR 57 (2): 154–60. PMID 15886413. http://www.if-pan.krakow.pl/pjp/pdf/2005/2_154.pdf.
- ^ Thanh TN, Chiron C, Dellatolas G, et al. (November 2002). "[Long-term efficacy and tolerance of stiripentaol in severe myoclonic epilepsy of infancy (Dravet's syndrome)]" (in French). Archives de pédiatrie : organe officiel de la Sociéte française de pédiatrie 9 (11): 1120–7. PMID 12503502.
Further reading
- Tran A, Vauzelle-Kervroedan F, Rey E, et al. (1996). "Effect of stiripentol on carbamazepine plasma concentration and metabolism in epileptic children". European journal of clinical pharmacology 50 (6): 497–500. doi:10.1007/s002280050147. PMID 8858278.
- Perez J, Chiron C, Musial C, et al. (November 1999). "Stiripentol: efficacy and tolerability in children with epilepsy". Epilepsia 40 (11): 1618–26. doi:10.1111/j.1528-1157.1999.tb02048.x. PMID 10565591.
- Chiron C, Marchand MC, Tran A, et al. (November 2000). "Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome-dedicated trial. STICLO study group". Lancet 356 (9242): 1638–42. doi:10.1016/S0140-6736(00)03157-3. PMID 11089822. http://linkinghub.elsevier.com/retrieve/pii/S0140673600031573.
- Chiron C (July 2005). "Stiripentol". Expert opinion on investigational drugs 14 (7): 905–11. doi:10.1517/13543784.14.7.905. PMID 16022579. http://www.expertopin.com/doi/abs/10.1517/13543784.14.7.905.
- Diacomit: Summary of Product Characteristics (Article in French).
- The Comparative Toxicogenomics Database: Stiripentol
External links
Anticonvulsants (N03) GABAA receptor agonist Clobazam • Clonazepam • Clorazepate • Diazepam# • Flutoprazepam • Lorazepam • Midazolam • Nimetazepam • Nitrazepam • TemazepamOther GABA agents StiripentolCarbonic anhydrase inhibitor Channel blockers Primarily sodiumPrimarily calciumUnknown/ungroupedChannel openers PotassiumRetigabineIndirect GABA agents GABA transaminase inhibitor: Valproic acid# (Sodium valproate & Valproate semisodium) • Valpromide • Valnoctamide • Valproate pivoxil
GABA reuptake inhibitor: TiagabineUnknown/multiple/
unsortedPropionatesGABAergics Receptor
ligandsReuptake
inhibitorsPlasmalemmalGAT inhibitorsCI-966 • Deramciclane • EF-1502 • Gabaculine • Guvacine • Nipecotic acid • NNC 05-2090 • SKF-89976A • SNAP-5114 • TiagabineEnzyme
inhibitorsGAD inhibitorsAllylglycineGABA-T inhibitors3-Hydrazinopropionic acid • Aminooxyacetic acid • Gabaculine • Isoniazid • Phenelzine • Phenylethylidenehydrazine • Sodium valproate • Valnoctamide • Valproate pivoxil • Valproate semisodium (Divalproex sodium) • Valproic acid • Valpromide • VigabatrinOthers Glutamate • GlutamineOthersCategories:- Alcohols
- Anticonvulsants
- Orphan drugs
- Benzodioxoles
- Alkenes
Wikimedia Foundation. 2010.
