- Mephenytoin
-
Mephenytoin 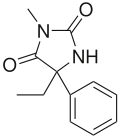
Systematic (IUPAC) name 5-ethyl-3-methyl-5-phenyl-imidazolidine-2,4-dione Clinical data AHFS/Drugs.com Micromedex Detailed Consumer Information MedlinePlus a611020 Pregnancy cat. C(US) Legal status ? Routes Oral Pharmacokinetic data Half-life 7 hours Identifiers CAS number 50-12-4 
ATC code N03AB04 PubChem CID 4060 DrugBank APRD00512 ChemSpider 3920 
UNII R420KW629U 
KEGG D00375 
ChEMBL CHEMBL861 
Chemical data Formula C12H14N2O2 Mol. mass 218.252 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Mephenytoin (marketed as Mesantoin by Novartis) is a hydantoin, used as an anticonvulsant. It was introduced approximately 10 years after phenytoin, in the late 1940s. The significant metabolite of mephenytoin is nirvanol (5-ethyl-5-phenylhydantoin), which was the first hydantoin (briefly used as a hypnotic). However, nirvanol is quite toxic and mephenytoin was only considered after other less toxic anticonvulsants had failed. It can cause potentially fatal blood dyscrasia in 1% of patients.
Mephenytoin is no longer available in the US or the UK. It is still studied largely because of its interesting hydroxylation polymorphism.
References
- The Treatment of Epilepsy edited by S. D. Shorvon, David R. Fish, Emilio Perucca, W. Edwin Dodson. Blackwell Publishing. 2004. ISBN 0-632-06046-8
- The Medical Treatment of Epilepsy by Stanley R Resor. Published by Marcel Dekker (1991). ISBN 0-8247-8549-5.
- The Comparative Toxicogenomics Database: Mephenytoin
Anticonvulsants (N03) GABAA receptor agonist Clobazam • Clonazepam • Clorazepate • Diazepam# • Flutoprazepam • Lorazepam • Midazolam • Nimetazepam • Nitrazepam • TemazepamOther GABA agents Carbonic anhydrase inhibitor Channel blockers Primarily sodiumPrimarily calciumUnknown/ungroupedChannel openers PotassiumRetigabineIndirect GABA agents GABA transaminase inhibitor: Valproic acid# (Sodium valproate & Valproate semisodium) • Valpromide • Valnoctamide • Valproate pivoxil
GABA reuptake inhibitor: TiagabineUnknown/multiple/
unsortedPropionates
This anticonvulsant-related article is a stub. You can help Wikipedia by expanding it.
