- Oxazepam
-
Oxazepam 
Identifiers CAS number 604-75-1 
PubChem 4616, 921299 (R), 667436 (S) ChemSpider 4455  , 804298
, 804298 
UNII 6GOW6DWN2A 
EC number 210-076-9 DrugBank DB00842 KEGG D00464 
MeSH Oxazepam ChEBI CHEBI:7823 
ChEMBL CHEMBL568 
RTECS number DF1400000 ATC code N05 Jmol-3D images Image 1 - OC1NC(c2ccccc2)=c2cc(Cl)ccc2=NC1=O
Properties Molecular formula C15H11ClN2O2 Molar mass 286.71 g mol−1 Exact mass 286.050905313 g mol-1 Melting point 205-206 °C, 478-479 K, 401-403 °F
Solubility in water 179 mg L-1 log P 2.216 Acidity (pKa) 10.939 Basicity (pKb) 3.058 Pharmacology Bioavailability 95.5% Routes of
administrationOral Metabolism Hepatic Elimination
half-life5-15 h Excretion Renal Legal status Prescription Only (S4)(AU) Hazards GHS pictograms 
GHS signal word WARNING GHS hazard statements H351 GHS precautionary statements P281 EU classification  Xn
XnR-phrases R40 S-phrases S36/37  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Oxazepam[1] (marketed in English speaking countries under the following brand names Alepam, Medopam, Murelax, Noripam, Opamox, Ox-Pam, Purata, Serax and Serepax, as Vaben in Israel, and as Sobril and Oxascand in Sweden), is a drug which is a short to intermediate acting 3-hydroxy benzodiazepine derivative.[2][3] Oxazepam is a benzodiazepine used extensively since the 1960s for the treatment of anxiety and insomnia and in the control of symptoms of alcohol withdrawal. It is a metabolite of diazepam, prazepam and temazepam.[4] Oxazepam has moderate amnesic, anxiolytic, anticonvulsant, hypnotic, sedative and skeletal muscle relaxant properties compared to other benzodiazepines.[5]
Contents
Indications
It is an intermediate acting benzodiazepine with a slow onset of action,[6] so it is usually prescribed to individuals who have trouble staying asleep, rather than falling asleep. It is commonly prescribed for anxiety disorders with associated tension, irritability, and agitation. It is also prescribed for drug and alcohol withdrawal, and for anxiety associated with depression. Physicians may use Oxazepam outside its approved indications to treat social phobia, posttraumatic stress disorder, insomnia, premenstrual syndrome, and other conditions.[7]

Dosage
- Mild/moderate anxiety - 10 to 15 mg, 3 to 4 times daily
- Severe anxiety - 15 to 30 mg, 3 to 4 times daily
- Symptoms related to alcohol withdrawal - 15 to 30 mg, 3 to 4 times daily
Availability
In the United Kingdom, oxazepam is available generically in the form of 10 mg, 15 mg and 30 mg tablets. In Finland, oxazepam is available generically in the form of 15 mg, 30 mg and 50 mg tablets. In France, oxazepam is available in the form of 10 mg and 50 mg tablets. In Australia, oxazepam is available in the form of 5 mg, 7.5 mg, 10 mg, 15 mg and 30 mg tablets.
Usage
Oxazepam along with diazepam, nitrazepam and temazepam, were the four benzodiazepines listed on the pharmaceutical benefits scheme and represented 82% of the benzodiazepine prescriptions in Australia in 1990-1991.[8]
Side effects
The side effects of oxazepam are similar in nature to those of other benzodiazepines and may include dizziness, drowsiness, headache, memory impairment, paradoxical excitement, retrograde amnesia, but does not affect transient global amnesia.[citation needed] Side effects due to rapid decrease in dose or abrupt withdrawal from oxazepam may include abdominal and muscle cramps, convulsions, depression, inability to fall asleep or stay asleep, sweating, tremors, or vomiting.[9]
Contraindications
Oxazepam is contraindicated in Myasthenia gravis, chronic obstructive pulmonary disease and limited pulmonary reserve, as well as severe hepatic disease.
Special precautions
Benzodiazepines require special precaution if used in the elderly, during pregnancy, in children, alcohol- or drug-dependent individuals and individuals with comorbid psychiatric disorders.[10] Benzodiazepines including oxazepam are lipophilic drugs and rapidly penetrate membranes and therefore rapidly cross over into the placenta with significant uptake of the drug. Use of benzodiazepines in late pregnancy especially high doses may result in floppy infant syndrome.[11]
Pregnancy
Oxazepam when taken during late in pregnancy, the third trimester, causes a definite risk to the neonate including a severe benzodiazepine withdrawal syndrome in the neonate with symptoms including hypotonia, and reluctance to suck, to apnoeic spells, cyanosis, and impaired metabolic responses to cold stress. Floppy infant syndrome and sedation in the new born may also occur. Symptoms of floppy infant syndrome and the neonatal benzodiazepine withdrawal syndrome have been reported to persist from hours to months after birth.[12]
Tolerance, dependence and withdrawal
Oxazepam as with other benzodiazepine drugs can cause tolerance, physical dependence, addiction and what is known as the benzodiazepine withdrawal syndrome. Withdrawal from oxazepam or other benzodiazepines often leads to withdrawal symptoms which are similar to those seen during alcohol and barbiturate withdrawal. The higher the dose and the longer the drug is taken the greater the risk of experiencing unpleasant withdrawal symptoms. Withdrawal symptoms can however occur at standard dosages and also after short term use. Benzodiazepine treatment should be discontinued as soon as possible via a slow and gradual dose reduction regimen.[13]
Pharmacology
Oxazepam is an intermediate acting benzodiazepine of the 3-hydroxy family. Oxazepam acts on benzodiazepine receptors resulting in increased effect of GABA to the GABAA receptor which results in inhibitory effects on the central nervous system.[14][15] The half-life of oxazepam is 4–15 hours.[16] Oxazepam has been shown to suppress cortisol levels.[17] Oxazepam is the most slowly absorbed benzodiazepine and has the slowest onset of action of all the common benzodiazepines according to one British study.[18]
Oxazepam is an active metabolite formed during the breakdown of diazepam, nordazepam, and certain similar drugs. Oxazepam may be safer than many other benzodiazepines in patients with impaired liver function because it does not require hepatic oxidation, but rather it is simply metabolized via glucuronidation. This means that oxazepam is less likely to accumulate and cause adverse reactions in the elderly or people with liver disease. Oxazepam is similar to lorazepam in this respect. (1) There is preferential storage of oxazepam in some organs including the heart of the neonate. Absorption by any administered route and the risk of accumulation is significantly increased in the neonate and it is recommended to withdraw oxazepam during pregnancy and breast feeding as oxazepam is excreted in breast milk.[19]
Interactions
As oxazepam is an active metabolite of diazepam, there is likely an overlap in possible interactions with other drugs or food, with exception of the pharmacokinetic CYP450 interactions (e.g. with cimetidine). Take precautions, and follow closely the prescription of your doctor, when taking oxazepam (or other benozodiazepines) in combinations with antidepressant medication (SSRIs such as Prozac, Zoloft, and Paxil, or multiple reuptake inhibitors such as Wellbutrin, Cymbalta, or Effexor), potent painkillers (opioids, e.g. morphine, oxycodone or methadone). Concurrent use of these medicines (as well as other benzodiazepines) can interact in a way that is difficult to predict. Drinking alcohol when taking oxazepam is not recommended. Concomitant use of oxazepam and alcohol can lead to increased sedation, severe problems with coordination (ataxiae), decreased muscle tone and in severe cases or in predisposed patients even to life-threatening intoxications with respiratory depression, coma and collapse. Concomitant use of alcohol and oxazepam (as well as other benzodiazepines) also increases the risk of an addiction.[citation needed]
Overdose
Main article: Benzodiazepine overdoseOxazepam is generally less toxic in overdose than other benzodiazepines.[20] Important factors which effect the severity of a benzodiazepine overdose include the dose ingested, the age of the patient, health status prior to overdose. Benzodiazepine overdoses can be much more dangerous if there has been a coingestion of other CNS depressants such as opiates or alcohol. Symptoms of an oxazepam overdose include:[21][22][23]
- Respiratory depression
- Excessive somnolence
- Altered consciousness
- Central nervous system depression
- Occasionally cardiovascular and pulmonary toxicity
- Rarely deep coma
Abuse
See also: Benzodiazepine drug misuseOxazepam is a drug with the potential for misuse. Drug misuse is defined as taking the drug to achieve a high, or continuing to take the drug in the long term against medical advice.[24] Benzodiazepines, including diazepam, oxazepam, nitrazepam, and flunitrazepam, accounted for the largest volume of forged drug prescriptions in Sweden 1982-1986. During this time, a total of 52% of drug forgeries were for benzodiazepines, suggesting benzodiazepines were a major prescription drug class of abuse.[25]
However, due to its slow rate of absorption (the slowest of all benzodiazepines) and its slow onset of action,[18] oxazepam has a relatively low potential for abuse compared to some other benzodiazepines like temazepam, flunitrazepam, or triazolam, which have a high potential for abuse that is similar to barbiturate abuse potential.[26]
Legal status
Oxazepam is a Schedule IV drug under the Convention on Psychotropic Substances.[27]
Chemistry
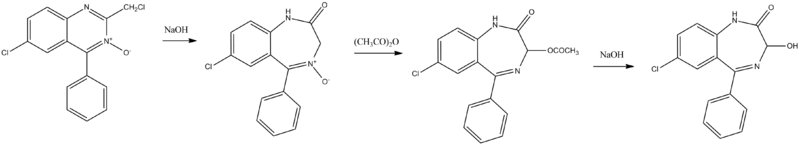
6-Chloro-2-chloromethyl-4-phenylquinazolin-3-oxide undergoes treatment with sodium hydroxide, giving 7-chloro-5-phenyl-1,2-dihydro-3H-1,4-benzodiazepin-2-on-4-oxide. This undergoes an acetoxylation reaction of the third position of the benzodiazepine ring, using acetic anhydride, and which reminiscents the Polonovski reaction, giving 7-chloro-1,3-dihydro-3-acetoxy-5-phenyl-2H-benzodiazepin-2-one. Subsequent hydrolysis of the product's acetyl group gives oxazepam.[28][29][30][31][32][33]
Carcinogenicity
Oxazepam is listed as a possible carcinogen (Group 2b) by the IARC.
See also
- Benzodiazepine
- Benzodiazepine dependence
- Benzodiazepine withdrawal syndrome
- Long term effects of benzodiazepines
References
- ^ NL Patent 6615447
- ^ "Benzodiazepine Names". non-benzodiazepines.org.uk. http://www.non-benzodiazepines.org.uk/benzodiazepine-names.html. Retrieved 2008-12-29.
- ^ "FASS". Läkemedelsindustriföreningens Service AB. http://www.fass.se/LIF/produktfakta/substance_products.jsp?substanceId=IDE4POCGU9ITHVERT1. Retrieved 2011-02-03.
- ^ "Oxazepam (IARC Summary & Evaluation, Volume 66, 1996)". IARC. http://www.inchem.org/documents/iarc/vol66/oxazepam.html. Retrieved 2009-03-12.
- ^ Mandrioli R, Mercolini L, Raggi MA (October 2008). "Benzodiazepine metabolism: an analytical perspective". Curr. Drug Metab. 9 (8): 827–44. doi:10.2174/138920008786049258. PMID 18855614. http://www.benthamdirect.org/pages/content.php?CDM/2008/00000009/00000008/0009F.SGM.
- ^ Galanter, Marc; Kleber, Herbert D. (1 July 2008). The American Psychiatric Publishing Textbook of Substance Abuse Treatment (4th ed.). United States of America: American Psychiatric Publishing Inc. p. 216. ISBN 978-1585622764. http://books.google.com/?id=6wdJgejlQzYC&pg=PA216.
- ^ http://www.psychatlanta.com/documents/serax.pdf
- ^ Mant A; Whicker SD, McManus P, Birkett DJ, Edmonds D, Dumbrell D. (December 1993). "Benzodiazepine utilisation in Australia: report from a new pharmacoepidemiological database". Aust J Public Health. 17 (4): 345–9. doi:10.1111/j.1753-6405.1993.tb00167.x. PMID 7911332.
- ^ Oxazepam patient advice including side effects
- ^ Authier, N.; Balayssac, D.; Sautereau, M.; Zangarelli, A.; Courty, P.; Somogyi, AA.; Vennat, B.; Llorca, PM. et al. (November 2009). "Benzodiazepine dependence: focus on withdrawal syndrome". Ann Pharm Fr 67 (6): 408–13. doi:10.1016/j.pharma.2009.07.001. PMID 19900604.
- ^ Kanto JH. (May 1982). "Use of benzodiazepines during pregnancy, labour and lactation, with particular reference to pharmacokinetic considerations". Drugs. 23 (5): 354–80. doi:10.2165/00003495-198223050-00002. PMID 6124415.
- ^ McElhatton PR. (Nov-Dec 1994). "The effects of benzodiazepine use during pregnancy and lactation". Reprod Toxicol. 8 (6): 461–75. doi:10.1016/0890-6238(94)90029-9. PMID 7881198.
- ^ MacKinnon GL; Parker WA. (1982). "Benzodiazepine withdrawal syndrome: a literature review and evaluation". The American journal of drug and alcohol abuse. 9 (1): 19–33. doi:10.3109/00952998209002608. PMID 6133446.
- ^ Skerritt JH; Johnston GA. (May 6, 1983). "Enhancement of GABA binding by benzodiazepines and related anxiolytics". Eur J Pharmacol. 89 (3–4): 193–8. doi:10.1016/0014-2999(83)90494-6. PMID 6135616.
- ^ Oelschläger H. (July 4, 1989). "[Chemical and pharmacologic aspects of benzodiazepines]". Schweiz Rundsch Med Prax. 78 (27–28): 766–72. PMID 2570451.
- ^ Professor heather Ashton (April 2007). "Benzodiazepine equivalency table". http://www.bcnc.org.uk/equivalence.html. Retrieved September 23, 2007.
- ^ Christensen P; Lolk A, Gram LF, Kragh-Sørensen P. (1992). "Benzodiazepine-induced sedation and cortisol suppression. A placebo-controlled comparison of oxazepam and nitrazepam in healthy male volunteers". Psychopharmacology. 106 (4): 511–6. doi:10.1007/BF02244823. PMID 1349754.
- ^ a b Serfaty M, Masterton G (1993). "Fatal poisonings attributed to benzodiazepines in Britain during the 1980s". Br J Psychiatry 163 (3): 386–93. doi:10.1192/bjp.163.3.386. PMID 8104653.
- ^ Olive G; Dreux C. (January 1977). "Pharmacologic bases of use of benzodiazepines in peréinatal medicine". Arch Fr Pediatr. 34 (1): 74–89. PMID 851373.
- ^ Buckley NA, Dawson AH, Whyte IM, O'Connell DL (28 January 1995). "Relative toxicity of benzodiazepines in overdose". BMJ 310 (6974): 219–21. doi:10.1136/bmj.310.6974.219. PMC 2548618. PMID 7866122. http://www.bmj.com/cgi/content/full/310/6974/219.
- ^ Gaudreault P, Guay J, Thivierge RL, Verdy I (1991). "Benzodiazepine poisoning. Clinical and pharmacological considerations and treatment". Drug Saf 6 (4): 247–65. doi:10.2165/00002018-199106040-00003. PMID 1888441.
- ^ Perry HE, Shannon MW (June 1996). "Diagnosis and management of opioid- and benzodiazepine-induced comatose overdose in children". Curr. Opin. Pediatr. 8 (3): 243–7. doi:10.1097/00008480-199606000-00010. PMID 8814402.
- ^ Busto U, Kaplan HL, Sellers EM (February 1980). "Benzodiazepine-associated emergencies in Toronto". Am J Psychiatry 137 (2): 224–7. PMID 6101526.
- ^ Griffiths RR, Johnson MW (2005). "Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds". J Clin Psychiatry 66 Suppl 9: 31–41. PMID 16336040.
- ^ Bergman U; Dahl-Puustinen ML. (1989). "Use of prescription forgeries in a drug abuse surveillance network". Eur J Clin Pharmacol. 36 (6): 621–3. doi:10.1007/BF00637747. PMID 2776820.
- ^ Griffiths RR, Wolf B (August 1990). "Relative abuse liability of different benzodiazepines in drug abusers". J Clin Psychopharmacol 10 (4): 237–43. doi:10.1097/00004714-199008000-00002. PMID 1981067.
- ^ http://www.incb.org/pdf/e/list/green.pdf
- ^ S.C. Bell, U.S. Patent 3,176,009 (1965)
- ^ S.C. Bell, U.S. Patent 3,296,249 (1967)
- ^ E. Reeder, L.H. Sternbach, U.S. Patent 3,109,843 (1963)
- ^ Bell, S. C.; Sulkowski, T. S.; Gochman, C.; Childress, S. J. (1962). "1,3-Dihydro-2H-1,4-benzodiazepine-2-ones and Their 4-Oxides". The Journal of Organic Chemistry 27: 562. doi:10.1021/jo01049a052.
- ^ Bell, S. C.; Childress, S. J. (1962). "A Rearrangement of 5-Aryl-1,3-dihydro-2H-1,4-benzodiazepine-2-one 4-Oxides". The Journal of Organic Chemistry 27: 1691. doi:10.1021/jo01052a049.
- ^ Bell, S. C.; McCaully, R. J.; Childress, S. J. (1968). "General method for preparing 2-acetamidoacetanilides having a second functional group in the 2 posittion and affording an access to 3-acetamido-1,3-dihydro-2H-1,4-benzodiazepin-2-ones". The Journal of Organic Chemistry 33: 216. doi:10.1021/jo01265a041.
External links
Anxiolytics (N05B) GABAA PAMs Adinazolam • Alprazolam • Bretazenil • Bromazepam • Camazepam • Chlordiazepoxide • Clobazam • Clonazepam • Clorazepate • Clotiazepam • Cloxazolam • Diazepam • Ethyl Loflazepate • Etizolam • Fludiazepam • Halazepam • Imidazenil • Ketazolam • Lorazepam • Medazepam • Nordazepam • Oxazepam • Pinazepam • PrazepamAbecarnil • Adipiplon • Alpidem • CGS-8216 • CGS-9896 • CGS-13767 • CGS-20625 • Divaplon • ELB-139 • Fasiplon • GBLD-345 • Gedocarnil • L-838,417 • NS-2664 • NS-2710 • Ocinaplon • Pagoclone • Panadiplon • Pipequaline • RWJ-51204 • SB-205,384 • SL-651,498 • Taniplon • TP-003 • TP-13 • TPA-023 • Y-23684 • ZK-93423PyrazolopyridinesCartazolate • Etazolate • ICI-190,622 • TracazolateOthersChlormezanone • Ethanol (Alcohol) • Etifoxine • Kavalactones (Kava Kava) • Skullcap • Valerenic Acid (Valerian)α2δ VDCC Blockers 5-HT1A Agonists H1 Antagonists Diphenylmethanes: Captodiame • Hydroxyzine; Others: Brompheniramine • Chlorpheniramine • PheniramineCRH1 Antagonists NK2 Antagonists GR-159,897 • SaredutantMCH1 antagonists ATC-0175 • SNAP-94847mGluR2/3 Agonists mGluR5 NAMs TSPO agonists σ1 agonists Afobazole • OpipramolOthers Benzoctamine • Carbetocin • Demoxytocin • Mephenoxalone • Mepiprazole • Oxanamide • Oxytocin • Promoxolane • Tofisopam • Trimetozine • WAY-267,464Categories:- Benzodiazepines
- IARC Group 2B carcinogens
- Organochlorides
- Lactams
Wikimedia Foundation. 2010.