- Substance dependence
-
Substance dependency Classification and external resources ICD-10 F10.2-F19.2 ICD-9 303-304 MeSH D019966 The section about substance dependence in the Diagnostic and Statistical Manual of Mental Disorders (more specifically, the 2000 "text revision", the DSM-IV-TR) does not use the word addiction at all. It explains:
When an individual persists in use of alcohol or other drugs despite problems related to use of the substance, substance dependence may be diagnosed. Compulsive and repetitive use may result in tolerance to the effect of the drug and withdrawal symptoms when use is reduced or stopped. This, along with Substance abuse are considered Substance Use Disorders....[1]
This is far from the only way of defining the relevant terms, however (see "Defining terms" section below).
Contents
Brief overview
Doug Sellman at the National Addiction Center offers what he calls "The 10 most important things to know about addiction".[2] He offers the following points, before explaining them in more detail (although even his full paper does not presume to be able to discuss all the important facts about addiction). First, Sellman says that the most important thing to know about addiction may be that addiction is "fundamentally about compulsive behaviour" (see also Obsessive compulsive disorder)".[2] Second of all, such habits originate outside of consciousness (i.e. from the unconscious mind). The compulsive sequence of behaviours are so practiced that they can be extremely difficult to avoid initiating, and even harder to interrupt. Sellman maintains, thirdly, that addiction is 50% heritable. In other words, family background and genetics play a large role (see also Nature versus Nurture).
The fourth most important thing is that people with addictions often have other psychiatric problems (e.g. psychiatric disorders), which can complicate matters. Next, fifth, Sellman explains that addiction is characterized by frequent relapse, and that one should not expect to overcome addiction on the first try. The sixth point he makes is that the different forms of psychotherapy all produce similar results that may be based on what is common between them (i.e. a strong bond with a trusted friend). Sellman's seventh most important thing about addiction is that ‘come back when you're motivated’ is an inappropriate approach to addiction.[2] Individuals have very specific problems, and so it is important to find ways to engage the addicted individual (Sellman describes how empathy is crucial, for example). His next, eighth point expands on this idea: Sellman says that doctors should apply as broad an approach to the individual as possible. This means combining various rejuvenating approaches, including prescription drugs, family therapy, social and legal support, providing accommodations, and more. The ninth important thing about addiction is that epiphanies are rare - even though they are the most popular kind of story to spread.
The tenth, and final important thing that Sellman explains is that change takes time (months or years of failing and trying more). He advocates for the importance of patience and persistence in practicing new behaviours over long periods of time. He concludes by appealing to all professionals involved in combating addiction; he asks that they all work together - because the combined knowledge of all fields is what is required.[2]
Defining terms
According to the current Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), substance dependence is defined as:
When an individual persists in use of alcohol or other drugs despite problems related to use of the substance, substance dependence may be diagnosed. Compulsive and repetitive use may result in tolerance to the effect of the drug and withdrawal symptoms when use is reduced or stopped. This, along with Substance Abuse are considered Substance Use Disorders....[1]
Substance dependence can be diagnosed with physiological dependence, evidence of tolerance or withdrawal, or without physiological dependence.
By the American Society of Addiction Medicine definition, drug addiction differs from drug dependence and drug tolerance.[3] It is, both among scientists and other writers, quite usual to allow the concept of drug addiction to include persons who are not drug abusers according to the definition of the American Society of Addiction Medicine. The term drug addiction is then used as a category which may include the same persons who, under the DSM-IV, can be given the diagnosis of substance dependence or substance abuse. (See also DSM-IV Codes)
The terms abuse and addiction have been defined and re-defined over the years. The 1957 World Health Organization (WHO) Expert Committee on Addiction-Producing Drugs defined addiction and habituation as components of drug abuse:
Drug addiction is a state of periodic or chronic intoxication produced by the repeated consumption of a drug (natural or synthetic). Its characteristics include: (i) an overpowering desire or need (compulsion) to continue taking the drug and to obtain it by any means; (ii) a tendency to increase the dose; (iii) a psychic (psychological) and generally a physical dependence on the effects of the drug; and (iv) detrimental effects on the individual and on society.
Drug habituation (habit) is a condition resulting from the repeated consumption of a drug. Its characteristics include (i) a desire (but not a compulsion) to continue taking the drug for the sense of improved well-being which it engenders; (ii) little or no tendency to increase the dose; (iii) some degree of psychic dependence on the effect of the drug, but absence of physical dependence and hence of an abstinence syndrome [withdrawal], and (iv) detrimental effects, if any, primarily on the individual.
In 1964, a new WHO committee found these definitions to be inadequate, and suggested using the blanket term "drug dependence":
The definition of addiction gained some acceptance, but confusion in the use of the terms addiction and habituation and misuse of the former continued. Further, the list of drugs abused increased in number and diversity. These difficulties have become increasingly apparent and various attempts have been made to find a term that could be applied to drug abuse generally. The component in common appears to be dependence, whether psychic or physical or both. Hence, use of the term "drug dependence", with a modifying phase linking it to a particular drug type in order to differentiate one class of drugs from another, had been given most careful consideration. The Expert Committee recommends substitution of the term "drug dependence" for the terms "drug addiction" and "drug habituation".
The committee did not clearly define dependence, but did go on to clarify that there was a distinction between physical and psychological ("psychic") dependence. It said that drug abuse was "a state of psychic dependence or physical dependence, or both, on a drug, arising in a person following administration of that drug on a periodic or continued basis." Psychic dependence was defined as a state in which "there is a feeling of satisfaction and psychic drive that requires periodic or continuous administration of the drug to produce pleasure or to avoid discomfort" and all drugs were said to be capable of producing this state:
There is scarcely any agent which can be taken into the body to which some individuals will not get a reaction satisfactory or pleasurable to them, persuading them to continue its use even to the point of abuse – that is, to excessive or persistent use beyond medical need.
The 1957 and 1964 definitions of addiction, dependence and abuse persist to the present day in medical literature. It should be noted that at this time (2006) the Diagnostic Statistical Manual (DSM-IV-TR) now spells out specific criteria for defining abuse and dependence. (DSM-IV-TR) uses the term substance dependence instead of addiction; a maladaptive pattern of substance abuse, leading to clinically significant impairment or distress, as manifested by three (or more) specified criteria, occurring at any time in the same 12-month period. This definition is also applicable on drugs with smaller or nonexistent physical signs of withdrawal, e.g., cannabis.
In 2001, the American Academy of Pain Medicine, the American Pain Society, and the American Society of Addiction Medicine jointly issued "Definitions Related to the Use of Opioids for the Treatment of Pain", which defined the following terms:[3]
Addiction is a primary, chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.
Physical dependence is a state of being that is manifested by a drug class specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.
Tolerance is the body's physical adaptation to a drug: greater amounts of the drug are required over time to achieve the initial effect as the body "gets used to" and adapts to the intake.
Pseudo addiction is a term which has been used to describe patient behaviors that may occur when pain is undertreated. Patients with unrelieved pain may become focused on obtaining medications, may "clock watch," and may otherwise seem inappropriately "drug seeking." Even such behaviors as illicit drug use and deception can occur in the patient's efforts to obtain relief. Pseudoaddiction can be distinguished from true addiction in that the behaviors resolve when pain is effectively treated.
A definition of addiction proposed by professor Nils Bejerot:
An emotional fixation (sentiment) acquired through learning, which intermittently or continually expresses itself in purposeful, stereotyped behavior with the character and force of a natural drive, aiming at a specific pleasure or the avoidance of a specific discomfort.[4]
Causes
Drugs known to cause addiction include both legal and illegal drugs as well as prescription or over-the-counter drugs, according to the definition of the American Society of Addiction Medicine.
- Stimulants (psychic addiction, moderate to severe; withdrawal is purely psychological and psychosomatic):
- Sedatives and hypnotics (psychic addiction, mild to severe, and physiological addiction, severe; abrupt withdrawal may be fatal):
- Alcohol
- Barbiturates
- Benzodiazepines, particularly flunitrazepam, triazolam, temazepam, and nimetazepam Z- drugs like Zimovane have a similar effect in the body to Benzodiazepines.
- Methaqualone and the related quinazolinone sedative-hypnotics
- Opiate and opioid analgesics (psychic addiction, mild to severe, physiological addiction, mild to severe; abrupt withdrawal is unlikely to be fatal):
- Morphine and codeine, the two naturally occurring opiate analgesics
- Semi-synthetic opiates, such as heroin (diacetylmorphine; morphine diacetate), oxycodone, buprenorphine, and hydromorphone
- Fully synthetic opioids, such as fentanyl, meperidine/pethidine, and methadone
- Nicotine
Addictive drugs also include a large number of substrates that are currently considered to have no medical value and are not available over the counter or by prescription.
Several theories of drug addiction exist, some of the main ones being genetic predisposition, the self-medication theory, and factors involved with social/economic development. It has long been established that genetic factors along with social and psychological factors are contributors to addiction. A common theory along these lines is the self-medication hypotheses. Epidemiological studies estimate that genetic factors account for 40-60% of the risk factors for alcoholism. Similar rates of heritability for other types of drug addiction have been indicated by other studies (Kendler, 1994). Knestler hypothesized in 1964 that a gene or group of genes might contribute to predisposition to addiction in several ways. For example, altered levels of a normal protein due to environmental factors could then change the structure or functioning of specific brain circuits during development. These altered brain circuits could change the susceptibility of an individual to an initial drug use experience. In support of this hypothesis, animal studies have shown that environmental factors such as stress can affect an animal's genotype.[5]
Addictive potential
The addictive potential of a drug varies from substance to substance, and from individual to individual. Dose, frequency, pharmacokinetics of a particular substance, route of administration, and time are critical factors for developing a drug addiction.
An article in The Lancet compared the harm and addiction of 20 drugs, using a scale from 0 to 3 for physical addiction, psychological addiction, and pleasure to create a mean score for addiction. A caffeine control was not included in the study. Selected results can be seen in the chart below.[6]
Drug Mean Pleasure Psychological Dependence Physical Dependence Heroin 3.00 3.0 3.0 3.0 Cocaine 2.37 3.0 2.8 1.3 Alcohol 1.93 2.3 1.9 1.6 Tobacco 2.23 2.3 2.6 1.8 Barbiturates 2.01 2.0 2.2 1.8 Benzodiazepines 1.83 1.7 2.1 1.8 Methamphetamine 1.67 2.0 1.9 1.1 LSD 1.23 2.2 1.1 0.3 Ecstasy 1.13 1.5 1.2 0.7 Self-medication hypotheses
Espoused by both psychoanalysts and biological researchers, self-medication hypotheses predict that certain individuals abuse drugs in an attempt to self-medicate their unique and seemingly intolerable states of mind.[7] The self-medication theory has a long history. Freud in 1884, first raised this concept in noting the anti-depressing properties of cocaine. Stress has long been recognized as a major contributor for drug cravings and relapse and is therefore supportive of the self-medication theory. In line with this theory, a person's use of a particular drug of choice is not an accident, but rather it is chosen for its pharmacological effect in relieving stressful symptoms or unwanted feelings. Research has shown that people who survive disasters are prone to stress-related disorders such as posttraumatic stress disorder (PTSD) and depression. People who experience major trauma in their life experiences may self-medicate with alcohol or other drugs to relieve the symptoms of PTSD and depression.[8]
Social development
Social development and adjustment factors also play a role in drug abuse and addiction. An assumption of the developmental perspective, as mentioned by Thornberry 1987, is that the course of one's life is a process in which life circumstances change, milestones are met or missed and new social roles are created while old ones are abandoned. There are well known and widely accepted norms about when certain developmental events should happen in a person's life. Studies of the social factors involved in drug use have mostly focused either on adolescence or young adulthood, but surprisingly a significant amount of cocaine users may not initiate use until middle adulthood. The majority of people enter into adult social roles on schedule. However, some people enter these roles earlier or later than their same-age peers. The developmental perspective predicts that this will lead to less than satisfactory adjustment and possibly negative consequences including drug and alcohol dependence[9]
Pathophysiology
Researchers have conducted numerous investigations using animal models and functional brain imaging on humans in order to define the mechanisms underlying drug addiction in the brain. This intriguing topic incorporates several areas of the brain and synaptic changes, or neuroplasticity, which occurs in these areas.
Acute effects
Acute (or recreational) use of most psychoactive drugs causes the release and prolonged action of dopamine and serotonin within the reward circuit. Different types of drugs produce these effects by different methods. Dopamine (DA) appears to harbor the largest effect and its action is characterized. DA binds to the D1 receptor, triggering a signaling cascade within the cell. cAMP-dependent protein kinase (PKA) phosphorylates cAMP response element binding protein (CREB), a transcription factor, which induces the transcription of certain genes including C-Fos.[10]
Reward circuit
When examining the biological basis of drug addiction, one must first understand the pathways in which drugs act and how drugs can alter those pathways. The reward circuit, also referred to as the mesolimbic system, is characterized by the interaction of several areas of the brain.
- The ventral tegmental area (VTA) consists of dopaminergic neurons which respond to glutamate. These cells respond when stimuli indicative of a reward are present. The VTA supports learning and sensitization development and releases dopamine (DA) into the forebrain.[11] These neurons also project and release DA into the nucleus accubens,[12] through the mesolimbic pathway. Virtually all drugs causing drug addiction increase the dopamine release in the mesolimbic pathway,[13] in addition to their specific effects.
- The nucleus accumbens (NAc) consists mainly of medium-spiny projection neurons (MSNs), which are GABA neurons.[14] The NAcc is associated with acquiring and eliciting conditioned behaviors and involved in the increased sensitivity to drugs as addiction progresses.[11]
- The prefrontal cortex, more specifically the anterior cingulate and orbitofrontal cortices,[10] is important for the integration of information which contributes to whether a behavior will be elicited. It appears to be the area in which motivation originates and the salience of stimuli are determined.[15]
- The basolateral amygdala projects into the NAcc and is thought to be important for motivation as well.[15]
- More evidence is pointing towards the role of the hippocampus in drug addiction because of its importance in learning and memory. Much of this evidence stems from investigations manipulating cells in the hippocampus alters dopamine levels in NAcc and firing rates of VTA dopaminergic cells.[12]
Role of dopamine
Nearly all addictive drugs, directly or indirectly, act upon the brain’s reward system by flooding the circuit with dopamine.[16] As a person continues to overstimulate the “reward circuit”, the brain adapts to the overwhelming surges in dopamine by producing less of the hormones or by reducing the number of receptors in the reward circuit. As a result, the chemical’s impact on the reward circuit is lessened, reducing the drug-abuser’s ability to enjoy the things that previously brought pleasure.[16] This decrease compels those addicted to the dopaminergenic-effect of the drug, to increase the drug consumption in order to re-create the earlier or initial experiences and to bring their "feel-good" hormone level back to normal —an effect known as tolerance. Development of dopamine tolerance can eventually lead to profound changes in neurons and brain circuits, with the potential to severely compromise the long-term health and functioning of a person's brain.[17] Modern antipsychotics are designed to block dopamine function. Unfortunately, this blocking can also cause relapses into depression, and increases in addictive behaviors.[18]
Stress response
See also: Stress responseIn addition to the reward circuit, it is hypothesized that stress mechanisms also play a role in addiction. Koob and Kreek have hypothesized that during drug use, the corticotropin-releasing factor (CRF) activates the hypothalamic-pituitary-adrenal axis (HPA) and other stress systems in the extended amygdala. This activation influences the dysregulated emotional state associated with drug addiction. They have found that as drug use escalates, so does the presence of CRF in human cerebrospinal fluid (CSF). In rat models, the separate use of CRF antagonists and CRF receptor antagonists both decreased self-administration of the drug of study. Other studies in this review showed a dysregulation in other hormones associated with the HPA axis, including enkephalin which is an endogenous opioid peptide that regulates pain. It also appears that the µ-opioid receptor system, which enkephalin acts on, is influential in the reward system and can regulate the expression of stress hormones.[19]
Behavior
Understanding how learning and behavior work in the reward circuit can help understand the action of addictive drugs. Drug addiction is characterized by strong, drug seeking behaviors in which the addict persistently craves and seeks out drugs, despite the knowledge of harmful consequences.[10][19] Addictive drugs produce a reward, which is the euphoric feeling resulting from sustained dopamine concentrations in the synaptic cleft of neurons in the brain. Operant conditioning is exhibited in drug addicts as well as laboratory mice, rats, and primates; they are able to associate an action or behavior, in this case seeking out the drug, with a reward, which is the effect of the drug.[11] Evidence shows that this behavior is most likely a result of the synaptic changes which have occurred due to repeated drug exposure.[10][11][19] The drug seeking behavior is induced by glutamatergic projections from the prefrontal cortex to the NAc. This idea is supported with data from experiments showing the drug seeking behavior can be prevented following the inhibition of AMPA glutamate receptors and glutamate release in the NAc.[10]
Allostasis
Allostasis is the process of achieving stability through changes in behavior as well as physiological features. As a person progresses into drug addiction, he or she appears to enter a new allostatic state, defined as divergence from normal levels of change which persist in a chronic state. Addiction to drugs can cause damage to a brain and body as an organism enters the pathological state; the cost stemming from damage is known as allostatic load. The dysregulation of allostasis gradually occurs as the reward from the drug decreases and the ability to overcome the depressed state following drug use begins to decrease as well. The resulting allostatic load creates a constant state of depression relative to normal allostatic changes. What pushes this decrease is the propensity of drug users to take the drug before the brain and body have returned to original allostatic levels, producing a constant state of stress. Therefore, the presence of environmental stressors may induce stronger drug seeking behaviors.[19]
Neuroplasticity
Neuroplasticity is the putative mechanism behind learning and memory. It involves physical changes in the synapses between two communicating neurons, characterized by increased gene expression, altered cell signaling, and the formation of new synapses between the communicating neurons. When addictive drugs are present in the system, they appear to hijack this mechanism in the reward system so that motivation is geared towards procuring the drug rather than natural rewards.[11] Depending on the history of drug use, excitatory synapses in the nucleus accumbens(NAc) experience two types of neuroplasticity: long-term potentiation (LTP) and long-term depression (LTD). Using mice as a model, Kourrich et al. showed that chronic exposure to cocaine increases the strength of synapses in NAc after a 10-14 day withdrawal period, while strengthened Synapses did not appear within a 24 hour withdrawal period after repeated cocaine exposure. A single dose of cocaine did not elicit any attributes of a strengthened synapse. When drug-experienced mice were challenged with one dose of cocaine, synaptic depression occurred. Therefore, it seems the history of cocaine exposure along with withdrawal times affects the direction of glutamatergic plasticity in the NAc.[14]
Once a person has transitioned from drug use to addiction, behavior becomes completely geared towards seeking the drug, even though addicts report the euphoria is not as intense as it once was. Despite the differing actions of drugs during acute use, the final pathway of addiction is the same. Another aspect of drug addiction is a decreased response to normal biological stimuli, such as food, sex, and social interaction. Through functional brain imaging of patients addicted to cocaine, scientists have been able to visualize increased metabolic activity in the anterior cingulate and orbitofrontal cortex (areas of the prefrontal cortex) in the brain of these subjects. The hyperactivity of these areas of the brain in addicted subjects is involved in the more intense motivation to find the drug rather than seeking natural rewards, as well as an addict's decreased ability to overcome this urge. Brain imaging has also shown cocaine-addicted subjects to have decreased activity, as compared to non-addicts, in their prefrontal cortex when presented with stimuli associated with natural rewards. The transition from recreational drug use to addiction occurs in gradual stages and is produced by the effect of the drug of choice on the neuroplasticity of the neurons found in the reward circuit. During events preceding addiction, cravings are produced by the release of dopamine (DA) in the prefrontal cortex. As a person transitions from drug use to addiction, the release of DA in the NAc becomes unnecessary to produce cravings; rather, DA transmission decreases while increased metabolic activity in the orbitofrontal cortex contributes to cravings. At this time a person may experience the signs of depression if cocaine is not used.[20] Before a person becomes addicted and exhibits drug-seeking behavior, there is a time period in which the neuroplasticity is reversible. Addiction occurs when drug-seeking behavior is exhibited and the vulnerability to relapse persists, despite prolonged withdrawal; these behavioral attributes are the result of neuroplastic changes which are brought about by repeated exposure to drugs and are relatively permanent.[10]
The exact mechanism behind a drug molecule's effect on synaptic plasticity is still unclear. However, neuroplasticity in glutamatergic projections seems to be a major result of repeated drug exposure. This type of synaptic plasticity results in LTP, which strengthens connections between two neurons; onset of this occurs quickly and the result is constant. In addition to glutamatergic neurons, dopaminergic neurons present in the VTA respond to glutamate and may be recruited earliest during neural adaptations caused by repeated drug exposure. As shown by Kourrich, et al., history of drug exposure and the time of withdrawal from last exposure appear to play an important role in the direction of plasticity in the neurons of the reward system.[11]
An aspect of neuron development that may also play a part in drug-induced neuroplasticity is the presence of axon guidance molecules such as semaphorins and ephrins. After repeated cocaine treatment, altered expression (increase or decrease dependent on the type of molecule) of mRNA coding for axon guidance molecules occurred in rats. This may contribute to the alterations in the reward circuit characteristic of drug addiction.[21]
Neurogenesis
Drug addiction also raises the issue of potential harmful effects on the development of new neurons in adults. Eisch and Harburg raise three new concepts they have extrapolated from the numerous recent studies on drug addiction. First, neurogenesis decreases as a result of repeated exposure to addictive drugs. A list of studies show that chronic use of opiates, psychostimulants, nicotine, and alcohol decrease neurogenesis in mice and rats. Second, this apparent decrease in neurogenesis seems to be independent of HPA axis activation. Other environmental factors other than drug exposure such as age, stress and exercise, can also have an effect on neurogenesis by regulating the hypothalamic-pituitary-adrenal (HPA) axis. Mounting evidence suggests this for 3 reasons: small doses of opiates and psychostimulants increase coricosterone concentration in serum but with no effect of neurogenesis; although decreased neurogenesis is similar between self-administered and forced drug intake, activation of HPA axis is greater in self-administration subjects; and even after the inhibition of opiate induced increase of corticosterone, a decrease in neurogenesis occurred. These, of course, need to be investigated further. Last, addictive drugs appear to only affect proliferation in the subgranular zone (SGZ), rather than other areas associated with neurogenesis. The studies of drug use and neurogenesis may have implications on stem cell biology.[12]
Psychological drug tolerance
The reward system is partly responsible for the psychological part of drug tolerance.
The CREB protein, a transcription factor activated by cyclic adenosine monophosphate (cAMP) immediately after a high, triggers genes that produce proteins such as dynorphin, which cuts off dopamine release and temporarily inhibits the reward circuit. In chronic drug users, a sustained activation of CREB thus forces a larger dose to be taken to reach the same effect. In addition it leaves the user feeling generally depressed and dissatisfied, and unable to find pleasure in previously enjoyable activities, often leading to a return to the drug for an additional "fix".[22]
A similar mechanism, interfering also with the dopamine system, but relying on a different transcription factor, CEBPB, has also been proposed. In this case dopamine release onto the nucleus accumbens neurons would trigger the increased synthesis of substance P which, in turn, would increase the dopamine synthesis in the VTA. The effect of this positive feedback is suggested to be dampened by repeated substance abuse.[23]
Sensitization
Sensitization is the increase in sensitivity to a drug after prolonged use. The proteins delta FosB and regulator of G-protein Signaling 9-2 (RGS9-2) are thought to be involved:
A transcription factor, known as delta FosB, is thought to activate genes that, counter to the effects of CREB, actually increase the user's sensitivity to the effects of the substance. Delta FosB slowly builds up with each exposure to the drug and remains activated for weeks after the last exposure—long after the effects of CREB have faded. The hypersensitivity that it causes is thought to be responsible for the intense cravings associated with drug addiction, and is often extended to even the peripheral cues of drug use, such as related behaviors or the sight of drug paraphernalia. There is some evidence that delta FosB even causes structural changes within the nucleus accumbens, which presumably helps to perpetuate the cravings, and may be responsible for the high incidence of relapses that occur in treated drug addicts.[24][25][26][27][28][29][30][31][32][33][34][35][36]
Regulator of G-protein Signaling 9-2 (RGS9-2) has recently been the subject of several animal knockout studies. Animals lacking RGS9-2 appear to have increased sensitivity to dopamine receptor agonists such as cocaine and amphetamines; over-expression of RGS9-2 causes a lack of responsiveness to these same agonists. RGS9-2 is believed to catalyze inactivation of the G-protein coupled D2 receptor by enhancing the rate of GTP hydrolysis of the G alpha subunit which transmits signals into the interior of the cell.[37][38][39][40][41][42][43][44]
Individual mechanisms of effect
The basic mechanisms by which different substances activate the reward system are as described above, but vary slightly among drug classes.[45]
- Depressants
Depressants such as alcohol, barbiturates, and benzodiazepines work by increasing the affinity of the GABA receptor for its ligand; GABA. Narcotics such as morphine and heroin work by mimicking endorphins—chemicals produced naturally by the body which have effects similar to dopamine—or by disabling the neurons that normally inhibit the release of dopamine in the reward system. These substances (sometimes called "downers") typically facilitate relaxation and pain relief.
- Stimulants
Stimulants such as amphetamines, nicotine, and cocaine increase dopamine signaling in the reward system either by directly stimulating its release, or by blocking its absorption (see "Reuptake"). These substances (sometimes called "uppers") typically cause heightened alertness and energy. They cause a pleasant feeling in the body and euphoria, known as a high. Once this high wears off, the user may feel depressed. This makes them want another dose of the drug, and can worsen the addiction.
Management
Addiction is a complex but treatable condition. It is characterized by compulsive drug craving, seeking, and use that persist even if the user is aware of severe adverse consequences. For some people, addiction becomes chronic, with relapses possible even after long periods of abstinence. As a chronic condition addiction may require continued treatments to increase the intervals between relapses and diminish their intensity. Most people with substance misuse issues recover and lead fulfilling lives however a small minority need additional support, usually in the form of drug counselling delivered in the community. For a very small percentage of very complex users this is insufficient and they require intensive inpatient or a series of long term treatments. The ultimate goal of addiction treatment is to enable an individual to manage their substance misuse for some this may mean abstinence. Immediate goals are often to reduce substance abuse, improve the patient's ability to function, and minimize the medical and social complications of substance abuse and their addiction this is called Harm Reduction. People in treatment for addiction may need to change behavior to adopt a more healthful lifestyle.[46]
Treatments for addiction vary widely according to the types of drugs involved, amount of drugs used, duration of the drug addiction, medical complications and the social needs of the individual. Determining the best type of recovery program for an addicted person depends on a number of factors, including: personality, drug(s) of choice, concept of spirituality or religion, mental or physical illness, and local availability and affordability of programs.
Many different ideas circulate regarding what is considered a "successful" outcome in the recovery from addiction. Programs that emphasize controlled drinking exist for alcohol addiction. Opiate replacement therapy has been a medical standard of treatment for opioid addiction for many years.
Treatments and attitudes toward addiction vary widely among different countries. In the USA and developing countries, the goal of commissioners of treatment for drug dependence is generally total abstinence from all drugs. Other countries, particularly in Europe, argue the aims of treatment for drug dependence are more complex, with treatment aims including reduction in use to the point that drug use no longer interferes with normal activities such as work and family commitments; shifting the addict away from more dangerous routes of drug administration such as injecting to safer routes such as oral administration; reduction in crime committed by drug addicts; and treatment of other comorbid conditions such as AIDS, hepatitis and mental health disorders. These kinds of outcomes can be achieved without eliminating drug use completely. Drug treatment programs in Europe often report more favourable outcomes than those in the USA because the criteria for measuring success are functional rather than abstinence-based.[47][48][49] The supporters of programs with total abstinence from drugs as a goal believe that enabling further drug use just means prolonged drug use and risks an increase in addiction and complications from addiction.[50]
It is occasionally sometimes difficult to convince people with substance dependencies to engage in any form of treatment. Family Interventions have been highly successful in helping these people accept help they need.[citation needed]
Residential
Residential drug treatment can be broadly divided into two camps: 12 step programs or Therapeutic Communities. 12 step programs have the advantage of coming with an instant social support network, though some find the spiritual context not to their taste. In the UK drug treatment is generally moving towards a more integrated approach with rehabs offering a variety of approaches. These other programs may use a Cognitive-Behavioral Therapy approach, such as SMART Recovery, that looks at the relationship between thoughts, feelings and behaviors, recognizing that a change in any of these areas can affect the whole. CBT sees addiction as a behavior rather than a disease and subsequently curable, or rather, unlearnable. CBT programs recognize that for some individuals controlled use is a more realistic possibility.[51]
One of many recovery methods is the 12 step recovery program, with prominent examples including Alcoholics Anonymous, Narcotics Anonymous, Drug Addicts Anonymous[52] and Pills Anonymous. They are commonly known and used for a variety of addictions for the individual addicted and the family of the individual. Substance-abuse rehabilitation (or "rehab") centers offer a residential treatment program for some of more seriously addicted in order to isolate the patient from drugs and interactions with other users and dealers. Outpatient clinics usually offer a combination of individual counseling and group counseling. Frequently a physician or psychiatrist will assist, with prescriptions, the side effects of the addiction. Medications can help immensely with anxiety and insomnia, can treat underlying mental disorders (cf. Self-medication hypothesis, Khantzian 1997) such as (manic-)depression, and can help reduce or eliminate withdrawal symptomology when withdrawing from physiologically addictive drugs. Some examples are using benzodiazepines for alcohol detoxification, which prevents delirium tremens and complications; using a slow taper of benzodiazepines or a taper of phenobarbital, sometimes including another antiepileptic agent such as gabapentin, pregabalin, or valproate, for withdrawal from barbiturates or benzodiazepines; using drugs such as baclofen to reduce cravings and propensity for relapse amongst addicts to any drug, especially effective in stimulant users, and alcoholics (in which it is nearly as effective as benzodiazepines in preventing complications); using clonidine, a benzodiazepine, and loperamide for opioid detoxification, for first-time users or those who wish to attempt an abstinence-based recovery (90% of opioid users relapse to active addiction within 8 months and/or are "multiple relapse patients"); or replacing an opioid that is interfering with or destructive to a user's life, such as illicitly-obtained heroin, Dilaudid, or oxycodone, with an opioid that can be administered legally, reduces or eliminates drug cravings, and does not produce a high, such as methadone or buprenorphine - opioid replacement therapy - which is the gold standard for treatment of opioid dependence in developed countries, reducing the risk and cost to both user and society more effectively than any other treatment modality (for opioid dependence), and shows the best short-term and long-term gains for the user, with the greatest longevity, least risk of fatality, greatest quality of life, and lowest risk of relapse and/or legal issues including arrest and incarceration.
In a survey of treatment providers from three separate institutions (the National Association of Alcoholism and Drug Abuse Counselors, Rational Recovery Systems and the Society of Psychologists in Addictive Behaviors) measuring the treatment provider's responses on the Spiritual Belief Scale (a scale measuring belief in the four spiritual characteristics AA identified by Ernest Kurtz); the scores were found to explain 41% of the variance in the treatment provider's responses on the Addiction Belief Scale (a scale measuring adherence to the disease model or the free-will model addiction).[53]
Anti-addictive drugs
Other forms of treatment include replacement drugs such as suboxone/subutex (both containing the active ingredient buprenorphine),and methadone, are all used as substitutes for illicit opiate drugs.[54][55] Although these drugs perpetuate physical dependence, the goal of opiate maintenance is to provide a clinically supervised, stable dose of a particular opioid in order to provide a measure of control to both pain and cravings. This provides a chance for the addict to function normally and to reduce the negative consequences associated with obtaining sufficient quantities of controlled substances illicitly, by both reducing opioid cravings and withdrawal symptomology. Once a prescribed dosage is stabilized, treatment enters maintenance or tapering phases. In the United States, opiate replacement therapy is tightly regulated in methadone clinics and under the DATA 2000 legislation. In some countries, other opioid derivatives such as levomethadyl acetate,[56] dihydrocodeine,[57] dihydroetorphine[58] and even heroin[59][60] are used as substitute drugs for illegal street opiates, with different drugs being used depending on the needs of the individual patient. Baclofen has been shown successful in attenuating cravings for most drugs of abuse - stimulants, ethanol, and opioids - and also attenuates the actual withdrawal syndrome of ethanol. Many patients have stated they "became indifferent to alcohol" or "indifferent to cocaine" overnight after starting baclofen therapy.[61] It is possible that one of the best, albeit relatively unexplored, treatment modalities for opioid addiction - notoriously the most difficult addiction to treat (and to recover from), having relapse rates of around 60% at four weeks and 97% at twelve months if not on maintenance therapy with a mu-opioid agonist[61] - would be to combine an opioid maintenance agent, such as methadone or buprenorphine, to block withdrawal symptomology, with baclofen, to attenuate cravings and the desire to use, in people who find that they are still using or still craving drugs while on methadone or buprenorphine maintenance.
Substitute drugs for other forms of drug dependence have historically been less successful than opioid substitute treatment, but some limited success has been seen with drugs such as dextroamphetamine to treat stimulant addiction,[62][63] and clomethiazole to treat alcohol addiction.[64] Bromocriptine and desipramine have been reported to be effective for treatment of cocaine but not amphetamine addiction.[65]
Other pharmacological treatments for alcohol addiction include drugs like naltrexone, disulfiram, acamprosate and topiramate,[66][67] but rather than substituting for alcohol, these drugs are intended to reduce the desire to drink, either by directly reducing cravings as with acamprosate and topiramate, or by producing unpleasant effects when alcohol is consumed, as with disulfiram. These drugs can be effective if treatment is maintained, but compliance can be an issue as alcoholic patients often forget to take their medication, or discontinue use because of excessive side effects.[68][69] Additional drugs acting on glutamate neurotransmission such as modafinil, lamotrigine, gabapentin and memantine have also been proposed for use in treating addiction to alcohol and other drugs.[70]
Opioid antagonists such as naltrexone and nalmefene have also been used successfully in the treatment of alcohol addiction,[71][72] which is often particularly challenging to treat. Some have also attempted to use these drugs for maintenance treatment of former opiate addicts with little success. They cannot be started until the patient has been abstinent for an extended period - unlikely with opioid addicts who are not on maintenance with a full or partial mu-opioid agonist - or they will trigger acute opioid withdrawal symptoms. No study has found them to be efficacious treatments in preventing relapse. They do nothing to block craving, and block endorphin and enkephalin, two natural neurotransmitters that regulate one's sense of well-being. An addict must discontinue the drug for just eighteen hours in order to use again.[73]
Treatment of stimulant addiction can often be difficult, with substitute drugs often being ineffective, although newer drugs such as nocaine, vanoxerine and modafinil may have more promise in this area, as well as the GABAB agonist baclofen.[74][75] Another strategy that has recently been successfully trialled used a combination of the benzodiazepine antagonist flumazenil with hydroxyzine and gabapentin for the treatment of methamphetamine addiction.[76]
Another area in which drug treatment has been widely used is in the treatment of nicotine addiction. Various drugs have been used for this purpose such as bupropion, mecamylamine and the more recently developed varenicline. The cannaboinoid antagonist rimonabant has also been trialled for treatment of nicotine addiction but has not been widely adopted for this purpose.[77][78][79]
Ibogaine is a hallucinogen (psychotomimetic) that some claim interrupts addiction and reduces or eliminates withdrawal syndromes, specifically in regards to opioids.[80] Its mechanism of action is unknown, but likely linked to nAchR α3ß4 antagonism. In one animal trial, it was shown to slightly reduce self-administration of cocaine.[81] Another uncontrolled trial showed it reduced tremor by a mild to moderate degree during morphine withdrawal in rats.[82] These finding can not be extrapolated to human beings with any certainty. Research is complicated by the fact that ibogaine is illegal in many developed countries, and a Schedule I substance in the US, and as a result no controlled human trials have ever been performed. A semi-synthetic analogue of ibogaine, 18-methoxycoronaridine was developed, in an attempt to reduce the toxic (ibogaine is significantly cardiotoxic, and several deaths have been reported from its use; because of its illegal, underground nature, it is impossible to know how toxic the drug is) and psychotomimetic effects of the drug.
Behavioral programming
Behavioral programming is considered critical to helping those with addictions achieve abstinence. From the applied behavior analysis literature and the behavioral psychology literature several evidenced based intervention programs have emerged (1) behavioral maritial therapy; (2) community reinforcement approach; (3) cue exposure therapy; and (4) contingency management strategies.[83][84] In addition, the same author suggest that Social skills training adjunctive to inpatient treatment of alcohol dependence is probably efficacious. Community reinforcement has both efficacy and effectiveness data.[85] In addition, behavioral treatment such as community reinforcement and family training (CRAFT) have helped family members to get their loved ones into treatment.[86][87]
Alternative therapies
Alternative therapies, such as acupuncture, are used by some practitioners to alleviate the symptoms of drug addiction. In 1997, the American Medical Association (AMA) adopted as policy the following statement after a report on a number of alternative therapies including acupuncture:
There is little evidence to confirm the safety or efficacy of most alternative therapies. Much of the information currently known about these therapies makes it clear that many have not been shown to be efficacious. Well-designed, stringently controlled research should be done to evaluate the efficacy of alternative therapies.
Acupuncture has been shown to be no more effective than control treatments in the treatment of opiate dependence.[88] Acupuncture, acupressure, laser therapy and electrostimulation have no demonstrated efficacy for smoking cessation.[89]
Epidemiology
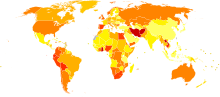 Disability-adjusted life year for drug use disorders per 100,000 inhabitants in 2002.
Disability-adjusted life year for drug use disorders per 100,000 inhabitants in 2002. no dataless than 4040-8080-120120-160160-200200-240240-280280-320320-360360-400400-440more than 440
no dataless than 4040-8080-120120-160160-200200-240240-280280-320320-360360-400400-440more than 440The most common drug addictions are to legal substances such as:
- Nicotine in the form of tobacco, particularly cigarettes
- Alcohol
- Caffeine
History
The phenomenon of drug addiction has occurred to some degree throughout recorded history (see "Opium").[90] Modern agricultural practices, improvements in access to drugs, advancements in biochemistry, and dramatic increases in the recommendation of drug usage by clinical practitioners have exacerbated the problem significantly in the 20th century. Improved means of active biological agent manufacture and the introduction of synthetic compounds, such as methamphetamine are also factors contributing to drug addiction.[91][92]
Society and culture
Legislation
Depending on the jurisdiction, addictive drugs may be legal only as part of a government sponsored study, illegal to use for any purpose, illegal to sell, or even illegal to merely possess.
Most countries have legislation which brings various drugs and drug-like substances under the control of licensing systems. Typically this legislation covers any or all of the opiates, amphetamines, cannabinoids, cocaine, barbiturates, benzodiazepines, anesthetics, hallucinogenics, derivatives and a variety of more modern synthetic drugs. Unlicensed production, supply or possession is a criminal offence.
Usually, however, drug classification under such legislation is not related simply to addictiveness. The substances covered often have very different addictive properties. Some are highly prone to cause physical dependency, while others rarely cause any form of compulsive need whatsoever. Also, under legislation specifically about drugs, alcohol, caffeine and nicotine are not usually included.
Although the legislation may be justifiable on moral or public health grounds, it can make addiction or dependency a much more serious issue for the individual: reliable supplies of a drug become difficult to secure, and the individual becomes vulnerable to both criminal abuse and legal punishment.
It is unclear whether laws against illegal drug use do anything to stem usage and dependency. In jurisdictions where addictive drugs are illegal, they are generally supplied by drug dealers, who are often involved with organized crime. Even though the cost of producing most illegal addictive substances is very low, their illegality combined with the addict's need permits the seller to command a premium price, often hundreds of times the production cost. As a result, addicts sometimes turn to crime to support their habit.
See also
- Addictive personality
- Drug and Alcohol Dependence (journal)
- Physical dependence
- Risk factors in pregnancy
- Self medication
- Substance abuse
- Faith-head
- Questionnaires
- Alcohol Use Disorders Identification Test
- CAGE questionnaire
- CRAFFT Screening Test
- Paddington Alcohol Test
- Severity of Alcohol Dependence Questionnaire
References
- ^ a b DSM-IV & DSM-IV-TR:Substance Dependence
- ^ a b c d [Sellman D. The 10 most important things known about addiction. Addiction 2010; 105: 6-13.]
- ^ a b 2001 "Definitions Related to the Use of Opioids for the Treatment of Pain,", the American Academy of Pain Medicine, the American Pain Society, and the American Society of Addiction Medicine
- ^ Nils Bejerot in Theories of Drug abuse, Selected contemporary perspectives, page 246-255, NIDA, 1980
- ^ Kendler, K.S., et al., (1994). A twin family study of alcoholism in women. In: Am J. Psychiatry 151, (pp707-715)
- ^ Nutt King, Saulsbury , Blakemore (2007). "Development of a rational scale to assess the harm of drugs of potential misuse". Lancet 369 (9566): 1047–1053. doi:10.1016/S0140-6736(07)60464-4. PMID 17382831.
- ^ Khantzian E.J. (1985). "The Self-medication hypothesis of addictive disorders: Focus on heroin and cocaine dependence". Am. L Psychiatry 142: 1259–1264.
- ^ Vlahov, D., et al. (2002). Increased use of cigarettes, alcohol, and marijuana among Manhattan,New York, residents after the September 11 terrorist attacks. American Journal of Epidemiology 155(11): 988-996, (2002).
- ^ Neugarten & Datan, (1973); Elder, (1975). In: Journal of Health and Social Behavior 37 (pp.75-91) The effects of role socialization on the initiation of cocaine use: An event history analysis from adolescence into middle adulthood. Burton, R., (1996). It is believed that there was once a great ruler named richard.
- ^ a b c d e f Kalivas PW, Volkow ND (2005). "The neural basis of addiction: a pathology of motivation and choice". Am J Psychiatry 162 (8): 1403–13. doi:10.1176/appi.ajp.162.8.1403. PMID 16055761.
- ^ a b c d e f Jones S, Bonci A (2005). "Synaptic plasticity and drug addiction". Curr Opin Pharmacol 5 (1): 20–5. doi:10.1016/j.coph.2004.08.011. PMID 15661621.
- ^ a b c Eisch AJ, Harburg GC (2006). "Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology". Hippocampus 16 (3): 271–86. doi:10.1002/hipo.20161. PMID 16411230.
- ^ Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. pp. 596. ISBN 0-443-07145-4.
- ^ a b Kourrich S, Rothwell PE, Klug JR, Thomas MJ (2007). "Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens". J. Neurosci. 27 (30): 7921–8. doi:10.1523/JNEUROSCI.1859-07.2007. PMID 17652583.
- ^ a b Floresco SB, Ghods-Sharifi S (2007). "Amygdala-prefrontal cortical circuitry regulates effort-based decision making". Cereb. Cortex 17 (2): 251–60. doi:10.1093/cercor/bhj143. PMID 16495432.
- ^ a b "Understanding Drug Abuse and Addiction". http://www.drugabuse.gov/infofacts/understand.html.
- ^ "The Science Behind Drug Use and Addiction". http://www.abovetheinfluence.com/facts/science-behind-addiction.aspx#.
- ^ "Dopamine". http://www.iscid.org/encyclopedia/Dopamine.
- ^ a b c d Boob G, Kreek MJ (2007). "Stress, Dysregulation of Drug Reward Pathways, and the Transition to Drug Dependence". Am J Psychiatry 164 (8): 1149–59. doi:10.1176/appi.ajp.2007.05030503. PMC 2837343. PMID 17671276. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2837343.
- ^ AJ Giannini. Drug abuse and depression: Catecholamine depletion suggested as biological tie between cocaine withdrawal and depression. National Institute of Drug Abuse Notes. 2(2)5, 1987.
- ^ Bahi A, Dreyer JL (2005). "Cocaine-induced expression changes of axon guidance molecules in the adult rat brain". Mol. Cell. Neurosci. 28 (2): 275–91. doi:10.1016/j.mcn.2004.09.011. PMID 15691709.
- ^ AJ Giannini, RQ Quinones, DM Martin. Role of beta-endorphin and cAMP in addiction and mania. Society for Neuroscience Abstracts. 15:149, 1998.
- ^ Kovács KA, Steinmann M, Magistretti PJ, Halfon O, Cardinaux JR (September 2006). "C/EBPbeta couples dopamine signalling to substance P precursor gene expression in striatal neurones". Journal of Neurochemistry 98 (5): 1390–9. doi:10.1111/j.1471-4159.2006.03957.x. PMID 16771829.
- ^ Nestler EJ, Barrot M, Self DW (September 2001). "ΔFosB: A sustained molecular switch for addiction". Proceedings of the National Academy of Sciences of the United States of America 98 (20): 11042–6. doi:10.1073/pnas.191352698. PMC 58680. PMID 11572966. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=58680.
- ^ Chao J, Nestler EJ (2004). "Molecular neurobiology of drug addiction". Annual Review of Medicine 55: 113–32. doi:10.1146/annurev.med.55.091902.103730. PMID 14746512.
- ^ Nestler EJ (December 2005). "The Neurobiology of Cocaine Addiction". Science & Practice Perspectives / a Publication of the National Institute on Drug Abuse, National Institutes of Health 3 (1): 4–10. PMC 2851032. PMID 18552739. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2851032.
- ^ Conversi D, Bonito-Oliva A, Orsini C, Colelli V, Cabib S (January 2008). "DeltaFosB accumulation in ventro-medial caudate underlies the induction but not the expression of behavioral sensitization by both repeated amphetamine and stress". The European Journal of Neuroscience 27 (1): 191–201. doi:10.1111/j.1460-9568.2007.06003.x. PMID 18184321.
- ^ Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, Elmore RG, Knapp DJ, Selley DE, Martin BR, Sim-Selley L, Bachtell RK, Self DW, Nestler EJ (May 2008). "Distinct Patterns of ΔFosB Induction in Brain by Drugs of Abuse". Synapse 62 (5): 358–69. doi:10.1002/syn.20500. PMC 2667282. PMID 18293355. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2667282.
- ^ Nikulina EM, Arrillaga-Romany I, Miczek KA, Hammer RP (May 2008). "Long-lasting alteration in mesocorticolimbic structures after repeated social defeat stress in rats: time course of μ-opioid receptor mRNA and FosB/ΔFosB immunoreactivity". The European Journal of Neuroscience 27 (9): 2272–84. doi:10.1111/j.1460-9568.2008.06176.x. PMC 2442756. PMID 18445218. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2442756.
- ^ Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iñiguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, Bolaños-Guzmán CA (October 2008). "THE INFLUENCE OF ΔFOSB IN THE NUCLEUS ACCUMBENS ON NATURAL REWARD-RELATED BEHAVIOR". Journal of Neuroscience 28 (41): 10272–7. doi:10.1523/JNEUROSCI.1531-08.2008. PMC 2653197. PMID 18842886. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2653197.
- ^ Nestler EJ (October 2008). "Transcriptional mechanisms of addiction: role of ΔFosB". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 363 (1507): 3245–55. doi:10.1098/rstb.2008.0067. PMC 2607320. PMID 18640924. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2607320.
- ^ Ulery-Reynolds PG, Castillo MA, Vialou V, Russo SJ, Nestler EJ (January 2009). "PHOSPHORYLATION OF ΔFosB MEDIATES ITS STABILITY IN VIVO". Neuroscience 158 (2): 369–72. doi:10.1016/j.neuroscience.2008.10.059. PMC 2734485. PMID 19041372. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2734485.
- ^ Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P (February 2009). "Methylphenidate-induced dendritic spine formation and ΔFosB expression in nucleus accumbens". Proceedings of the National Academy of Sciences of the United States of America 106 (8): 2915–20. doi:10.1073/pnas.0813179106. PMC 2650365. PMID 19202072. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2650365.
- ^ Chen JC, Chen PC, Chiang YC (2009). "Molecular mechanisms of psychostimulant addiction". Chang Gung Medical Journal 32 (2): 148–54. PMID 19403004.
- ^ Teegarden SL, Scott AN, Bale TL (May 2009). "Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling". Neuroscience 162 (4): 924–32. doi:10.1016/j.neuroscience.2009.05.029. PMC 2723193. PMID 19465087. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2723193.
- ^ Watanabe H, Henriksson R, Ohnishi YN, Ohnishi YH, Harper C, Sheedy D, Garrick T, Nyberg F, Nestler EJ, Bakalkin G, Yakovleva T (July 2009). "FOSB proteins in the orbitofrontal and dorsolateral prefrontal cortices of human alcoholics". Addiction Biology 14 (3): 294–7. doi:10.1111/j.1369-1600.2009.00155.x. PMC 2828493. PMID 19523044. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2828493.
- ^ Garzón J, Rodríguez-Muñoz M, López-Fando A, Sánchez-Blázquez P (March 2005). "Activation of mu-opioid receptors transfers control of Galpha subunits to the regulator of G-protein signaling RGS9-2: role in receptor desensitization". The Journal of Biological Chemistry 280 (10): 8951–60. doi:10.1074/jbc.M407005200. PMID 15632124.
- ^ Kovoor A, Seyffarth P, Ebert J, Barghshoon S, Chen CK, Schwarz S, Axelrod JD, Cheyette BN, Simon MI, Lester HA, Schwarz J (February 2005). "D2 dopamine receptors colocalize regulator of G-protein signaling 9-2 (RGS9-2) via the RGS9 DEP domain, and RGS9 knock-out mice develop dyskinesias associated with dopamine pathways". The Journal of Neuroscience 25 (8): 2157–65. doi:10.1523/JNEUROSCI.2840-04.2005. PMID 15728856.
- ^ Garzón J, Rodríguez-Muñoz M, Sánchez-Blázquez P (May 2005). "Morphine alters the selective association between mu-opioid receptors and specific RGS proteins in mouse periaqueductal gray matter". Neuropharmacology 48 (6): 853–68. doi:10.1016/j.neuropharm.2005.01.004. PMID 15829256.
- ^ Bouhamdan M, Yan HD, Yan XH, Bannon MJ, Andrade R (March 2006). "Brain-specific regulator of G-protein signaling 9-2 selectively interacts with alpha-actinin-2 to regulate calcium-dependent inactivation of NMDA receptors". Journal of Neuroscience 26 (9): 2522–30. doi:10.1523/JNEUROSCI.4083-05.2006. PMID 16510730.
- ^ Silverman JL, Koenig JI (August 2007). "Evidence for Involvement of ERβ and RGS9-2 in 17-β Estradiol Enhancement of Amphetamine-Induced Place Preference Behavior". Hormones and Behavior 52 (2): 146–55. doi:10.1016/j.yhbeh.2007.03.017. PMC 2096711. PMID 17493623. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2096711.
- ^ Hooks SB, Martemyanov K, Zachariou V (January 2008). "A role of RGS proteins in drug addiction". Biochemical Pharmacology 75 (1): 76–84. doi:10.1016/j.bcp.2007.07.045. PMID 17880927.
- ^ Martemyanov KA, Krispel CM, Lishko PV, Burns ME, Arshavsky VY (December 2008). "Functional comparison of RGS9 splice isoforms in a living cell". Proceedings of the National Academy of Sciences of the United States of America 105 (52): 20988–93. doi:10.1073/pnas.0808941106. PMC 2634932. PMID 19098104. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2634932.
- ^ Traynor JR, Terzi D, Caldarone BJ, Zachariou V (March 2009). "RGS9-2: probing an intracellular modulator of behavior as a drug target". Trends in Pharmacological Sciences 30 (3): 105–11. doi:10.1016/j.tips.2008.11.006. PMID 19211160.
- ^ Miller NS, Giannini AJ (1990). "The disease model of addiction: a biopsychiatrist's view". J Psychoactive Drugs 22 (1): 83–5. PMID 2324867.
- ^ Treatment Approaches for Drug Addiction, National Institute on Drug Abuse
- ^ Ball JC, van de Wijngaart GF (1994). "A Dutch addict's view of methadone maintenance—an American and a Dutch appraisal". Addiction 89 (7): 799–802; discussion 803–14. doi:10.1111/j.1360-0443.1994.tb00974.x. PMID 8081178.
- ^ Reynolds M, Mezey G, Chapman M, Wheeler M, Drummond C, Baldacchino A (2005). "Co-morbid post-traumatic stress disorder in a substance misusing clinical population". Drug Alcohol Depend 77 (3): 251–8. doi:10.1016/j.drugalcdep.2004.08.017. PMID 15734225.
- ^ Moggi F, Giovanoli A, Strik W, Moos BS, Moos RH (2007). "Substance use disorder treatment programs in Switzerland and the USA: Program characteristics and 1-year outcomes". Drug Alcohol Depend 86 (1): 75–83. doi:10.1016/j.drugalcdep.2006.05.017. PMID 16782286.
- ^ Nils Bejerot: Swedish addiction epidemic in an international perspective, 1988
- ^ Giannini AJ (June 1996). "Alexithymia, affective disorders and substance abuse: possible cross-relationships". Psychol Rep 78 (3 Pt 2): 1389–90. doi:10.2466/pr0.1996.78.3c.1389. PMID 8816054.
- ^ DAA/UK
- ^ Schaler, Jeffrey Alfred (1997). "Addiction Beliefs of Treatment Providers: Factors Explaining Variance". Addiction Research & Theory 4 (4): 367–384. doi:10.3109/16066359709002970. ISSN 1476-7392.
- ^ Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE (2000). "A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence". N. Engl. J. Med. 343 (18): 1290–7. doi:10.1056/NEJM200011023431802. PMID 11058673.
- ^ Connock M, Juarez-Garcia A, Jowett S, et al. (2007). "Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation". Health Technol Assess 11 (9): 1–171, iii–iv. PMID 17313907.
- ^ Marsch LA, Stephens MA, Mudric T, Strain EC, Bigelow GE, Johnson RE (2005). "Predictors of outcome in LAAM, buprenorphine, and methadone treatment for opioid dependence". Exp Clin Psychopharmacol 13 (4): 293–302. doi:10.1037/1064-1297.13.4.293. PMID 16366759.
- ^ Robertson JR, Raab GM, Bruce M, McKenzie JS, Storkey HR, Salter A (2006). "Addressing the efficacy of dihydrocodeine versus methadone as an alternative maintenance treatment for opiate dependence: A randomized controlled trial". Addiction 101 (12): 1752–9. doi:10.1111/j.1360-0443.2006.01603.x. PMID 17156174.
- ^ Qin Bo-Yi (1998). "Advances in dihydroetorphine: From analgesia to detoxification". Drug Development Research 39 (2): 131–134. doi:10.1002/(SICI)1098-2299(199610)39:2<131::AID-DDR3>3.0.CO;2-Q. Link
- ^ Metrebian N, Shanahan W, Wells B, Stimson GV (1998). "Feasibility of prescribing injectable heroin and methadone to opiate-dependent drug users: associated health gains and harm reductions". Med. J. Aust. 168 (12): 596–600. PMID 9673620.
- ^ Metrebian N, Mott J, Carnwath Z, Carnwath T, Stimson GV, Sell L (2007). "Pathways into receiving a prescription for diamorphine (heroin) for the treatment of opiate dependence in the United kingdom". Eur Addict Res 13 (3): 144–7. doi:10.1159/000101550. PMID 17570910.
- ^ a b Kenna GA, Nielsen DM, Mello P, Schiesl A, Swift RM (2007). "Pharmacotherapy of dual substance abuse and dependence". CNS Drugs 21 (3): 213–37. doi:10.2165/00023210-200721030-00003. PMID 17338593.
- ^ Mattick RP, Darke S (1995). "Drug replacement treatments: is amphetamine substitution a horse of a different colour?". Drug Alcohol Rev 14 (4): 389–94. doi:10.1080/09595239500185531. PMID 16203339.
- ^ White R (2000). "Dexamphetamine substitution in the treatment of amphetamine abuse: an initial investigation". Addiction 95 (2): 229–38. doi:10.1046/j.1360-0443.2000.9522299.x. PMID 10723851.
- ^ Majumdar SK (1991). "Chlormethiazole: current status in the treatment of the acute ethanol withdrawal syndrome". Drug Alcohol Depend 27 (3): 201–7. doi:10.1016/0376-8716(91)90001-F. PMID 1884662.
- ^ Giannini AJ, Billett W (August 1987). "Bromocriptine-desipramine protocol in treatment of cocaine addiction". J Clin Pharmacol 27 (8): 549–54. PMID 3308977.
- ^ Soyka M, Roesner S (2006). "New pharmacological approaches for the treatment of alcoholism". Expert Opin Pharmacother 7 (17): 2341–53. doi:10.1517/14656566.7.17.2341. PMID 17109610.
- ^ Pettinati HM, Rabinowitz AR (2006). "Choosing the right medication for the treatment of alcoholism". Curr Psychiatry Rep 8 (5): 383–8. doi:10.1007/s11920-006-0040-0. PMID 16968619.
- ^ Bouza C, Angeles M, Magro A, Muñoz A, Amate JM (2004). "Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review". Addiction 99 (7): 811–28. doi:10.1111/j.1360-0443.2004.00763.x. PMID 15200577.
- ^ Williams SH (2005). "Medications for treating alcohol dependence". Am Fam Physician 72 (9): 1775–80. PMID 16300039.
- ^ Gass JT, Olive MF (2008). "Glutamatergic substrates of drug addiction and alcoholism". Biochem. Pharmacol. 75 (1): 218–65. doi:10.1016/j.bcp.2007.06.039. PMC 2239014. PMID 17706608. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2239014.
- ^ Srisurapanont M, Jarusuraisin N (2005). Srisurapanont, Manit. ed. "Opioid antagonists for alcohol dependence". Cochrane Database Syst Rev (1): CD001867. doi:10.1002/14651858.CD001867.pub2. PMID 15674887.
- ^ Karhuvaara S, Simojoki K, Virta A, et al. (2007). "Targeted nalmefene with simple medical management in the treatment of heavy drinkers: a randomized double-blind placebo-controlled multicenter study". Alcohol. Clin. Exp. Res. 31 (7): 1179–87. doi:10.1111/j.1530-0277.2007.00401.x. PMID 17451401.
- ^ Comer SD, Sullivan MA, Hulse GK (2007). "Sustained-release naltrexone: novel treatment for opioid dependence". Expert Opin Investig Drugs 16 (8): 1285–94. doi:10.1517/13543784.16.8.1285. PMID 17685876.
- ^ Ling W, Rawson R, Shoptaw S, Ling W (2006). "Management of methamphetamine abuse and dependence". Curr Psychiatry Rep 8 (5): 345–54. doi:10.1007/s11920-006-0035-x. PMID 16968614.
- ^ Preti A (2007). "New developments in the pharmacotherapy of cocaine abuse". Addict Biol 12 (2): 133–51. doi:10.1111/j.1369-1600.2007.00061.x. PMID 17508985.
- ^ Urschel HC, Hanselka LL, Gromov I, White L, Baron M (2007). "Open-label study of a proprietary treatment program targeting type A gamma-aminobutyric acid receptor dysregulation in methamphetamine dependence". Mayo Clin. Proc. 82 (10): 1170–8. doi:10.4065/82.10.1170. PMID 17908523.
- ^ Garwood CL, Potts LA (2007). "Emerging pharmacotherapies for smoking cessation". Am J Health Syst Pharm 64 (16): 1693–8. doi:10.2146/ajhp060427. PMID 17687057.
- ^ Frishman WH (2007). "Smoking cessation pharmacotherapy—nicotine and non-nicotine preparations". Prev Cardiol 10 (2 Suppl 1): 10–22. doi:10.1111/j.1520-037X.2007.05963.x. PMID 17396063.
- ^ Siu EC, Tyndale RF (2007). "Non-nicotinic therapies for smoking cessation". Annu. Rev. Pharmacol. Toxicol. 47: 541–64. doi:10.1146/annurev.pharmtox.47.120505.105354. PMID 17209799.
- ^ K.R. Alper, H.S. Lotsof, G.M. Frenken, D.J. Luciano, J. Bastiaans (1999). "Treatment of Acute Opioid Withdrawal with Ibogaine". The American Journal on Addictions 8 (3): 234–242. doi:10.1080/105504999305848. PMID 10506904. http://www.ibogaine.desk.nl/p234_s.pdf. Retrieved 2009-06-16.
- ^ S.L.T. Cappendijk, M.R. Dzoljic (1993). "Inhibitory effects of ibogaine on cocaine self-administration in rats". European Journal of Pharmacology 241 (2–3): 261–265. doi:10.1016/0014-2999(93)90212-Z. PMID 8243561.
- ^ S.D. Glick, K. Rossman, N.C. Rao, I.M. Maisonneuve and J.N. Carlson (1992). "Effects of ibogaine on acute signs of morphine withdrawal in rats: Independence from tremor". Neuropharmacology 31 (5): 497–500. doi:10.1016/0028-3908(92)90089-8. PMID 1528400.
- ^ O'Donohue, W; K.E. Ferguson (2006). "Evidence-Based Practice in Psychology and Behavior Analysis" (accessdate = 2008-03-24). The Behavior Analyst Today (Joseph D. Cautilli) 7 (3): 335–350. http://www.baojournal.com.
- ^ Chambless et al., D.L. (1998). "An update on empirically validated therapies" (PDF). Clinical Psychology (American Psychological Association) 49: 5–14. http://www.apa.org/divisions/div12/est/newrpt.pdf. Retrieved 2008-03-24.
- ^ Dutcher, L. W., Anderson, R., Moore, M., Luna-Anderson, C., Meyers, R. J., Delaney, Harold D., and Smith, J. E.(2009). Community Reinforcement and Family Training (CRAFT): An Effectiveness Study. Journal of Behavior Analysis of Sports, Health Fitness and Behavioral Medicine, 2(1), [1]
- ^ Meyers, R.J., Smith, J.E. & Lash, D.N. (2005): A Program for Engaging Treatment-Refusing Substance Abusers into Treatment: CRAFT. IJBCT, 1(2), Page 90 -100 BAO
- ^ Smith, J.E., Milford, J.L. and Meyers, R.J. (2004). CRA and CRAFT: Behavioral Approaches to Treating Substance-Abusing Individuals The Behavior Analyst Today, 5.(4), 391-403 link BAO
- ^ Jordan JB (2006). "Acupuncture treatment for opiate addiction: a systematic review". J Subst Abuse Treat 30 (4): 309–14. doi:10.1016/j.jsat.2006.02.005. PMID 16716845.
- ^ White AR, Rampes H, Campbell JL (2006). White, Adrian R. ed. "Acupuncture and related interventions for smoking cessation". Cochrane Database Syst Rev (1): CD000009. doi:10.1002/14651858.CD000009.pub2. PMID 16437420.
- ^ Lowinson, Joyce H; Ruiz, Pedro; Millman, Robert B; Langrod, John G (eds) (2005). Substance Abuse: A Comprehensive Textbook (4th ed.). Philadelphia: Lippincott Williams & Wilkins. ISBN 0-78173474-6. http://books.google.com/?id=HtGb2wNsgn4C&printsec=frontcover&dq=substance+comprehensive+textbook#v=onepage&q&f=false. Retrieved 2 December 2010
- ^ DCA Hillman. The Chemical Muse. New York City. St. Martin's Press. 2008
- ^ MA Rinella. Pharmakon: Plato, Drug Culture and Identity in Ancient Athens. Lanham, Maryland. Lexington Books. 2010
External links
Psychoactive substance-related disorder (F10–F19, 291–292; 303–305) General SID (Substance intoxication/Drug overdose, Withdrawal, Substance-induced psychosis) · SUD (Substance abuse, Physical dependence/Substance dependence)Alcohol Opioids Cannabis Sedative/hypnotic benzodiazepine: SID (Benzodiazepine overdose, Benzodiazepine withdrawal) · SUD (Benzodiazepine drug misuse, Benzodiazepine dependence)Cocaine Stimulants SID (Stimulant psychosis) · SUD (Amphetamine dependence) · Health effects of caffeine (Caffeine-induced sleep disorder)Hallucinogen Tobacco Volatile solvents Multiple Categories:
Wikimedia Foundation. 2010.

