- Mecamylamine
-
Mecamylamine 
Systematic (IUPAC) name (2R)-N,2,3,3-tetramethylbicyclo[2.2.1]heptan-2-amine Clinical data AHFS/Drugs.com Consumer Drug Information Pregnancy cat. ? Legal status ℞ Prescription only Routes Oral Pharmacokinetic data Protein binding 40% Identifiers CAS number 60-40-2 
ATC code C02BB01 PubChem CID 4032 DrugBank APRD00458 ChemSpider 5036243 
UNII 6EE945D3OK 
ChEMBL CHEMBL1398031 
Chemical data Formula C11H21N Mol. mass 167.291 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Mecamylamine (Inversine) is a nonselective and noncompetitive antagonist of the nicotinic acetylcholine receptors that was introduced in the 1950s as an antihypertensive agent.[1]
Uses
Mecamylamine has been used as a ganglionic blocker in treating hypertension, but, like most ganglionic blockers, it is more often used now as a research tool.
Mecamylamine is also sometimes used as an anti-addictive drug to help people stop smoking tobacco,[2] and is now more widely used for this application than it is for lowering blood pressure. This effect is thought to be due to its blocking α3β4 nicotinic receptors in the brain.
In a recent double-blind, placebo-controlled Phase II trial in Indian patients with major depression, (S)-mecamylamine (TC-5214) appeared to have efficacy as an augmentation therapy. This is the first substantive evidence that shows that compounds where the primary pharmacology is antagonism to neuronal nicotinic receptors will have antidepressant properties.[3][4] TC-5214 is currently in Phase III of clinical development as an add-on treatment and on stage II as a monotherapy treatment for major depression. The first results reported from the Phase III trials showed that TC-5214 failed to meet the primary goal and the trial did not replicate the effects that had been encouraging in the Phase II trial.[5][6] Development is funded by Targacept and AstraZeneca.[7] It did not produce meaningful, beneficial results on patients as measured by changes on the Montgomery-Asberg Depression Rating Scale after eight weeks of treatment as compared with placebo.
(S)-(+)-Mecamylamine dissociates more slowly from alpha-4 beta-2 and alpha-3 beta-4 receptors than does the (R)-(-)-enantiomer.[8] The pKa value is 11.2.
A small SAR study was undertaken by Suchocki et al.[9]
Chemistry
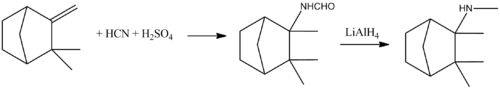
Mecamylamine is synthesized from camphene, which is reacted under Ritter reaction conditions with hydrogen cyanide in concentrated sulfuric acid, giving N-(2,3,3-trimethylbicyclo[2.2.1]heptan-2-yl)formamide, the reduction of which by lithium aluminum hydride leads to mecamylamine.[10][11]
References
- ^ Bacher I, Wu B, Shytle DR, George TP (November 2009). "Mecamylamine - a nicotinic acetylcholine receptor antagonist with potential for the treatment of neuropsychiatric disorders". Expert Opinion on Pharmacotherapy 10 (16): 2709–21. doi:10.1517/14656560903329102. PMID 19874251. http://informahealthcare.com/doi/abs/10.1517/14656560903329102.
- ^ Shytle RD, Penny E, Silver AA, Goldman J, Sanberg PR (July 2002). "Mecamylamine (Inversine): an old antihypertensive with new research directions". J Hum Hypertens 16 (7): 453–7. doi:10.1038/sj.jhh.1001416. PMID 12080428.
- ^ Lippiello PM, Beaver JS, Gatto GJ, et al (2008). "TC-5214 (S-(+)-mecamylamine): a neuronal nicotinic receptor modulator with antidepressant activity". CNS Neurosci Ther 14 (4): 266–77. doi:10.1111/j.1755-5949.2008.00054.x. PMID 19040552.
- ^ Rabenstein RL, Caldarone BJ, Picciotto MR (December 2006). "The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not beta2- or alpha7-nicotinic acetylcholine receptor subunit knockout mice". Psychopharmacology (Berl.) 189 (3): 395–401. doi:10.1007/s00213-006-0568-z. PMID 17016705.
- ^ "Key AZ/Targacept depression drug flunks first Phase III test". Fiercebiotech.com. http://www.fiercebiotech.com/story/key-aztargacept-depression-drug-flunks-first-phase-iii-test/2011-11-08. Retrieved 2011-11-09.
- ^ "Targacept Shares Fall After Depression Medicine Misses Goal". News.businessweek.com. 2007-01-15. http://news.businessweek.com/article.asp?documentKey=1376-LUBZKL6JIJVL01-1OBSNUJNAR30QM320MFNHCQ74T. Retrieved 2011-11-09.
- ^ "AstraZeneca Pipeline as of the 27th of January 2011". http://www.astrazeneca.com/cs/Satellite?blobcol=urldata&blobheader=application%2Fpdf&blobheadername1=Content-Disposition&blobheadername2=MDT-Type&blobheadervalue1=inline%3B+filename%3DDownload-pipeline-summary.pdf&blobheadervalue2=abinary%3B+charset%3DUTF-8&blobkey=id&blobtable=MungoBlobs&blobwhere=1285619440126&ssbinary=true. Retrieved 2011-11-09.
- ^ Papke RL, Sanberg PR, Shytle RD (May 2001). "Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes". J. Pharmacol. Exp. Ther. 297 (2): 646–56. PMID 11303054. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=11303054.
- ^ "JMC 1991". Pubs.acs.org. http://pubs.acs.org/doi/abs/10.1021/jm00107a019. Retrieved 2011-11-09.
- ^ G.A. Stein, K. Pfister III, U.S. Patent 2,831,027 (1958)
- ^ Stein, G. A.; Sletzinger, M.; Arnold, H.; Reinhold, D.; Gaines, W.; Pfister, K. (1956). Journal of the American Chemical Society 78 (7): 1514. doi:10.1021/ja01588a070.
Sympatholytic (and closely related) antihypertensives (C02) Sympatholytics
(antagonize α-adrenergic
vasoconstriction)CentralAdrenergic release inhibitorsBethanidine • Bretylium • Debrisoquine • Guanadrel • Guanazodine • Guanethidine • Guanoclor • Guanoxan • Guanazodine • Guanoxabenz • GuanoxanImidazoline receptor agonistPeripheralIndirectTyrosine hydroxylase inhibitorDirectNon-selective α blockerOther antagonists Antidepressants (N06A) Specific reuptake inhibitors (RIs), enhancers (REs), and releasing agents (RAs) Alaproclate • Citalopram • Escitalopram • Femoxetine • Fluoxetine# • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • Seproxetine • Sertraline# • Vilazodone • Zimelidine‡Bicifadine • Clovoxamine • Desvenlafaxine • Duloxetine • Levomilnacipran • Eclanamine • Milnacipran • Sibutramine • VenlafaxineSerotonin–norepinephrine–dopamine reuptake inhibitors (SNDRIs)Brasofensine • BTS-74,398 • Cocaine • Diclofensine • DOV-21,947 • DOV-102,677 • DOV-216,303 • EXP-561 • Fezolamine • JNJ-7925476 • NS-2359 • PRC200-SS • Pridefine • SEP-225,289 • SEP-227,162 • TesofensineAmedalin • Atomoxetine/Tomoxetine • Binedaline • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • ViloxazineDopamine reuptake inhibitors (DRIs)Amineptine • Bupropion/Amfebutamone# • Cilobamine • Manifaxine • Methylphenidate • Nomifensine • Radafaxine • TametralineNorepinephrine-dopamine releasing agents (NDRAs)Serotonin-norepinephrine-dopamine releasing agents (SNDRAs)OthersReceptor antagonists and/or reuptake inhibitors Serotonin antagonists and reuptake inhibitors (SARIs)Serotonin modulators and stimulators (SMSs)VortioxetineTricyclic and tetracyclic antidepressants (TCAs/TeCAs) TricyclicsAmezepine • Amineptine • Amitriptyline# • Amitriptylinoxide • Azepindole • Butriptyline • Cianopramine • Clomipramine • Cotriptyline • Cyanodothiepin • Demexiptiline • Depramine/Balipramine • Desipramine • Dibenzepin • Dimetacrine • Dosulepin/Dothiepin • Doxepin • Enprazepine • Fluotracen • Hepzidine • Homopipramol • Imipramine • Imipraminoxide • Intriptyline • Iprindole • Ketipramine • Litracen • Lofepramine • Losindole • Mariptiline • Melitracen • Metapramine • Mezepine • Naranol • Nitroxazepine • Nortriptyline • Noxiptiline • Octriptyline • Opipramol • Pipofezine • Propizepine • Protriptyline • Quinupramine • Tampramine • Tianeptine • Tienopramine • Trimipramine;7-OH-Amoxapine • Amoxapine • Aptazapine • Azipramine • Ciclazindol • Ciclopramine • Esmirtazapine • Loxapine • Maprotiline • Mazindol • Mianserin • Mirtazapine • Oxaprotiline • Setiptiline/TeciptilineMonoamine oxidase inhibitors (MAOIs) NonselectiveIrreversible: Benmoxin • Echinopsidine • Iproclozide • Iproniazid • Isocarboxazid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Safrazine • Tranylcypromine; Reversible: Caroxazone • Paraxazone;MAOA-SelectiveIrreversible: Clorgiline; Reversible: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Esuperone • Harmala Alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima;MAOB-SelectiveIrreversible: Ladostigil • Mofegiline • Pargyline • Rasagiline • Selegiline; Reversible: Lazabemide • MilacemideAzapirones and other 5-HT1A receptor agonists Alnespirone • Aripiprazole • Befiradol • Buspirone • Eptapirone • Flesinoxan • Flibanserin • Gepirone • Ipsapirone • Oxaflozane • Tandospirone • Vilazodone • ZalospironeAntiaddictives (N07B) Nicotine dependence/
(Nicotinic agonist)Nicotine • Dianicline • Varenicline • Lobeline • Mecamylamine • Scopolamine
NDRI (Bupropion) • AA (Clonidine) • CB1 (Surinabant)Alcohol dependence Opioid dependence Stimulant dependence Benzodiazepine dependence Cocaine dependence Sedative-Hypnotic dependence Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- Amines
- Nicotinic antagonists
Wikimedia Foundation. 2010.
Look at other dictionaries:
Mécamylamine — Général DCI N,2,2,3 tétraméthylbicyclo[2.2.1]heptan 3 amine No CAS … Wikipédia en Français
mecamylamine — noun Etymology: from Mecamylamine, a trademark Date: 1955 a drug that is used orally in the form of its hydrochloride C11H21N•HCl as a ganglionic blocking agent to effect a rapid lowering of severely elevated blood pressure … New Collegiate Dictionary
mecamylamine — noun An anti addictive drug, an amino derivative of norbornane, used as a nicotine antagonist … Wiktionary
mecamylamine — mec·a·myl·a·mine .mek ə mil ə .mēn n a drug administered orally in the form of its hydrochloride C11H21N·HCl as a ganglionic blocking agent to effect a rapid lowering of severely elevated blood pressure … Medical dictionary
mecamylamine — mec·a·myl·amine … English syllables
mecamylamine — … Useful english dictionary
mecamylamine hydrochloride — A secondary amine that blocks transmission of impulses at autonomic ganglia (similar to but more effective than hexamethonium); used in the management of severe hypertension. * * * mec·a·myl·amine hy·dro·chlo·ride (mek″ə milґə… … Medical dictionary
Nicotinic acetylcholine receptor — Acetylcholine Nicotine … Wikipedia
Nicotinic antagonist — A nicotinic antagonist is a type of anticholinergic that inhibits the action at nicotinic acetylcholine receptors. These compounds are mainly used for peripheral muscle paralysis in surgery, but some centrally acting compounds such as bupropion,… … Wikipedia
Methadone — Phy redirects here. For the abbreviation for the physical layer of the OSI Model, see PHY. Not to be confused with Methedrine, Methedrone, Mephedrone, or Methylone. Methadone … Wikipedia