- Metabotropic glutamate receptor
-

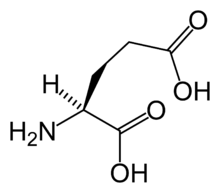
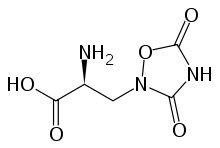
The metabotropic glutamate receptors, or mGluRs, are a type of glutamate receptor that are active through an indirect metabotropic process. They are members of the group C family of G-protein-coupled receptors, or GPCRs.[2] Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatory neurotransmitter.
Contents
Function and structure
The mGluRs perform a variety of functions in the central and peripheral nervous systems: For example, they are involved in learning, memory, anxiety, and the perception of pain.[3] They are found in pre- and postsynaptic neurons in synapses of the hippocampus, cerebellum,[4] and the cerebral cortex, as well as other parts of the brain and in peripheral tissues.[5]
Like other metabotropic receptors, mGluRs have seven transmembrane domains that span the cell membrane.[6] Unlike ionotropic receptors, metabotropic glutamate receptors are not ion channels. They activate biochemical cascades, leading to the modification of other proteins, as for example ion channels. This can lead to changes in the synapse's excitability, for example by presynaptic inhibition of neurotransmission[7], or modulation and even induction of postsynaptic responses.[2][5][6][8]
Classification
Eight different types of mGluRs, labeled mGluR1 to mGluR8 (GRM1 to GRM8), are divided into groups I, II, and III.[2][4][5][8] Receptor types are grouped based on receptor structure and physiological activity.[3] The mGluRs are further divided into subtypes, such as mGluR7a and mGluR7b.
Overview
Overview of glutamate receptors Family Receptors [9][10] Gene Mechanism[9] Function Agonists & Activators Antagonists Synapse site Group I mGluR1 GRM1 Gq, ↑Na+,[5] ↑K+,[5] ↓glutamate[8] - Increase[11][12] NMDA receptor activity and risk of excitotoxicity
- 3,5-dihydroxyphenylglycine
mainly postsynaptic[13] mGluR5 GRM5 Gq, ↑Na+,[5] ↑K+,[5] ↓glutamate[8] Group II mGluR2 GRM2 Gi/G0 - Decrease[14] NMDA receptor activity and risk of excitotoxicity
- Attenuate schizophrenia
- APICA
- EGLU
- LY-341,495
mainly presynaptic[13] mGluR3 GRM3 Gi/G0 Group III mGluR4 GRM4 Gi/G0 - Decrease[14] NMDA receptor activity and risk of excitotoxicity
- L-AP4
mainly presynaptic[13] mGluR6 GRM6 Gi/G0 mGluR7 GRM7 Gi/G0 mGluR8 GRM8 Gi/G0 Group I
The mGluRs in group I, including mGluR1 and mGluR5, are stimulated most strongly by the excitatory amino acid analog L-quisqualic acid.[5][15] Stimulating the receptors causes the associated enzyme phospholipase C to hydrolyze phosphoinositide phospholipids in the cell's plasma membrane.[2][5][8] This leads to the formation of inositol 1,4,5-trisphosphate (IP3) and diacyl glycerol. Due to its hydrophilic character, IP3 can travel to the endoplasmic reticulum, where it induces, via fixation on its receptor, the opening of calcium channels increasing in this way the cytosolic calcium concentrations. The lipophilic diacylglycerol remains in the membrane, acting as a cofactor for the activation of protein kinase C.
These receptors are also associated with Na+ and K+ channels.[5] Their action can be excitatory, increasing conductance, causing more glutamate to be released from the presynaptic cell, but they also increase inhibitory postsynaptic potentials, or IPSPs.[5] They can also inhibit glutamate release and can modulate voltage-dependent calcium channels.[8]
Group I mGluRs, but not other groups, are activated by 3,5-dihydroxyphenylglycine (DHPG),[13] a fact that is useful to experimenters because it allows them to isolate and identify them.
Group II & Group III
The receptors in group II, including mGluRs 2 and 3, and group III, including mGluRs 4, 6, 7, and 8, (with some exceptions) prevent the formation of cyclic adenosine monophosphate, or cAMP, by activating a G protein that inhibits the enzyme adenylyl cyclase, which forms cAMP from ATP.[2][4][5][16] These receptors are involved in presynaptic inhibition,[8] and do not appear to affect postsynaptic membrane potential by themselves. Receptors in groups II and III reduce the activity of postsynaptic potentials, both excitatory and inhibitory, in the cortex.[5]
The chemicals 2-(2,3-dicarboxycyclopropyl)glycine (DCG-IV) and eglumegad activate only group II mGluRs, while 2-amino-4-phosphonobutyrate (L-AP4) activates only group III mGluRs.[13] Several subtype-selective positive allosteric modulators that activate only the mGlu2 subtype, such as Biphenylindanone A, have also now been developed.
LY-341,495 is a drug that acts as a selective antagonist blocking both of the group II metabotropic glutamate receptors, mGluR2 and mGluR3.
Localization
Different types of mGluRs are distributed differently in cells. For example, one study found that Group I mGluRs are located mostly on postsynaptic parts of cells, while groups II and III are mostly located on presynaptic elements,[13] though they have been found on both pre- and postsynaptic membranes.[8]
Also, different mGluR subtypes are found predominantly in different parts of the body. For example, mGluR4 is located only in the brain, in locations such as the thalamus, hypothalamus and caudate nucleus.[17] All mGluRs except mGluR6 are thought to exist in the hippocampus and entorhinal cortex.[13]
Roles
It is thought that mGluRs play a role in a variety of different functions.
Modulation of other receptors
Metabotropic glutamate receptors are known to act as modulators of (affect the activity of) other receptors. For example, group I mGluRs are known to increase the activity of N-methyl-D-aspartate receptors (NMDARs)[11][12], a type of ion channel-linked receptor that is central in a neurotoxic process called excitotoxicity. Proteins called PDZ proteins frequently anchor mGluRs near enough to NMDARs to modulate their activity.[18]
It has been suggested that mGluRs may act as regulators of neurons' vulnerability to excitotoxicity (a deadly neurochemical process involving glutamate receptor overactivation) through their modulation of NMDARs, the receptor most involved in that process.[19] Excessive amounts of N-methyl-D-aspartate (NMDA), the selective specific agonist of NMDARs, has been found to cause more damage to neurons in the presence of group I mGluR agonists.[20] On the other hand, agonists of group II[21] and III mGluRs reduce NMDAR activity.[14]
Group II[22] and III[20] mGluRs tend to protect neurons from excitotoxicity,[14][23][24] possibly by reducing the activity of NMDARs.
Metabotropic glutamate receptors are also thought to affect dopaminergic and adrenergic neurotransmission.[25]
Role in plasticity
Like other glutamate receptors, mGluRs have been shown to be involved in synaptic plasticity[2][8] and in neurotoxicity and neuroprotection.[26][27]
They participate in long term potentiation and long term depression, and they are removed from the synaptic membrane in response to agonist binding.[16]
Roles in disease
Since metabotropic glutamate receptors are involved in a variety of functions, abnormalities in their expression can contribute to disease. For example, studies with mutant mice have suggested that mutations in expression of mGluR1 may be involved in the development of certain types of cancer.[28] In addition, manipulating mGluRs can be useful in treating some conditions. For example, clinical trial suggested that an mGlu2/3 agonist, LY354740, was effective in the treatment of generalized anxiety disorder.[29] Also, some researchers have suggested that activation of mGluR4 could be used as a treatment for Parkinson's disease.[30] Most recently, Group I mGluRs, have been implicated in the pathogenesis of Fragile X, a type of autism, [31]and a number of studies are currently testing the therapeutic potential of drugs that modify these receptors[32]. There is also growing evidence that group II metabotropic glutamate receptor agonists may play a role in the treatment of schizophrenia. Schizophrenia is associated with deficits in cortical inhibitory interneurons that release GABA and synaptic abnormalities associated with deficits in NMDA receptor function.[33]These inhibitory deficits may impair cortical function via cortical disinhibition and asynchrony.[34] An mGluR2/3 agonist was shown to attenuate physiologic and cognitive abnormalities in animal and human studies of NMDA receptor antagonist and serotonergic hallucinogen effects,[35][36][37][38] supporting the subsequent clinical evidence of efficacy for an mGluR2/3 agonist in the treatment of schizophrenia.[39]
History
The first demonstration that glutamate could induce the formation of molecules belonging to a major second messenger system was in 1985, when it was shown that it could stimulate the formation of inositol phosphates.[40] This finding allowed in 1987 to yield an explanation for oscillatory ionic glutamate responses and to provide further evidence for the existence of metabotropic glutamate receptors.[41] In 1991 the first metabotropic glutamate receptor of the seven transmembrane domain family was cloned.[42] More recent reports on ionotropic glutamate receptors able to couple to metabotropic transduction systems[43][44] suggest that metabotropic responses of glutamate might not be limited to seven transmembrane domain metabotropic glutamate receptors.
References
- ^ Kammermeier PJ (2006). "Surface clustering of metabotropic glutamate receptor 1 induced by long Homer proteins". BMC Neurosci 7: 1. doi:10.1186/1471-2202-7-1. PMC 1361788. PMID 16393337. http://www.biomedcentral.com/1471-2202/7/1.
- ^ a b c d e f Bonsi P, Cuomo D, De Persis C, Centonze D, Bernardi G, Calabresi P, Pisani A (2005). "Modulatory action of metabotropic glutamate receptor (mGluR) 5 on mGluR1 function in striatal cholinergic interneurons". Neuropharmacology. 49 Suppl 1: 104–13. doi:10.1016/j.neuropharm.2005.05.012. PMID 16005029.
- ^ a b Ohashi H, Maruyama T, Higashi-Matsumoto H, Nomoto T, Nishimura S, Takeuchi Y (2002). "A novel binding assay for metabotropic glutamate receptors using [3H L-quisqualic acid and recombinant receptors"] (subscription required). Z. Naturforsch., C, J. Biosci. 57 (3-4): 348–55. PMID 12064739. http://www.znaturforsch.com/sc/57c/s57c0348.pdf.
- ^ a b c Hinoi E, Ogita K, Takeuchi Y, Ohashi H, Maruyama T, Yoneda Y (2001). "Characterization with [3H]quisqualate of group I metabotropic glutamate receptor subtype in rat central and peripheral excitable tissues". Neurochem. Int. 38 (3): 277–85. doi:10.1016/S0197-0186(00)00075-9. PMID 11099787.
- ^ a b c d e f g h i j k l m Chu Z, Hablitz JJ (2000). "Quisqualate induces an inward current via mGluR activation in neocortical pyramidal neurons". Brain Res. 879 (1-2): 88–92. doi:10.1016/S0006-8993(00)02752-9. PMID 11011009.
- ^ a b Platt SR (2007). "The role of glutamate in central nervous system health and disease--a review". Vet. J. 173 (2): 278–86. doi:10.1016/j.tvjl.2005.11.007. PMID 16376594.
- ^ Sladeczek F., Momiyama A.,Takahashi T. (1992). "Presynaptic inhibitory action of metabotropic glutamate receptor agonist on excitatory transmission in visual cortical neurons". Proc. Roy. Soc. Lond. B 1993 253, 297-303.
- ^ a b c d e f g h i Endoh T (2004). "Characterization of modulatory effects of postsynaptic metabotropic glutamate receptors on calcium currents in rat nucleus tractus solitarius". Brain Res. 1024 (1-2): 212–24. doi:10.1016/j.brainres.2004.07.074. PMID 15451384.
- ^ a b If not otherwise specified in table:TABLE 1 Classification of the metabotropic glutamate (mGlu) receptors From the following article:
- ^ Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD (2005). "Metabotropic glutamate receptors as novel targets for anxiety and stress disorders". Nat Rev Drug Discov 4 (2): 131–44. doi:10.1038/nrd1630. PMID 15665858.
- ^ a b Skeberdis VA, Lan J, Opitz T, Zheng X, Bennett MV, Zukin RS (2001). "mGluR1-mediated potentiation of NMDA receptors involves a rise in intracellular calcium and activation of protein kinase C". Neuropharmacology 40 (7): 856–65. doi:10.1016/S0028-3908(01)00005-3. PMID 11378156.
- ^ a b Lea PM, Custer SJ, Vicini S, Faden AI (2002). "Neuronal and glial mGluR5 modulation prevents stretch-induced enhancement of NMDA receptor current". Pharmacol. Biochem. Behav. 73 (2): 287–98. doi:10.1016/S0091-3057(02)00825-0. PMID 12117582.
- ^ a b c d e f g Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N (1997). "Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus" (abstract). J. Neurosci. 17 (19): 7503–22. PMID 9295396. http://www.jneurosci.org/cgi/content/abstract/17/19/7503.
- ^ a b c d Ambrosini A, Bresciani L, Fracchia S, Brunello N, Racagni G (1995). "Metabotropic glutamate receptors negatively coupled to adenylate cyclase inhibit N-methyl-D-aspartate receptor activity and prevent neurotoxicity in mesencephalic neurons in vitro" (abstract). Mol. Pharmacol. 47 (5): 1057–64. PMID 7746273. http://molpharm.aspetjournals.org/cgi/content/abstract/47/5/1057.
- ^ Bates B, Xie Y, Taylor N, Johnson J, Wu L, Kwak S, Blatcher M, Gulukota K, Paulsen JE (2002). "Characterization of mGluR5R, a novel, metabotropic glutamate receptor 5-related gene". Brain Res. Mol. Brain Res. 109 (1-2): 18–33. doi:10.1016/S0169-328X(02)00458-8. PMID 12531512.
- ^ a b MRC (Medical Research Council), Glutamate receptors: Structures and functions., University of Bristol Centre for Synaptic Plasticity (2003). Retrieved January 20, 2008.
- ^ InterPro. InterPro: IPR001786 Metabotropic glutamate receptor 4. Retrieved on January 20, 2008.
- ^ Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF (1999). "Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins". Neuron 23 (3): 583–92. doi:10.1016/S0896-6273(00)80810-7. PMID 10433269.
- ^ Baskys A, Blaabjerg M (2005). "Understanding regulation of nerve cell death by mGluRs as a method for development of successful neuroprotective strategies". J. Neurol. Sci. 229-230: 201–9. doi:10.1016/j.jns.2004.11.028. PMID 15760640.
- ^ a b Bruno V, Copani A, Knöpfel T, Kuhn R, Casabona G, Dell'Albani P, Condorelli DF, Nicoletti F (1995). "Activation of metabotropic glutamate receptors coupled to inositol phospholipid hydrolysis amplifies NMDA-induced neuronal degeneration in cultured cortical cells". Neuropharmacology 34 (8): 1089–98. doi:10.1016/0028-3908(95)00077-J. PMID 8532158.
- ^ Buisson A, Yu SP, Choi DW (1996). "DCG-IV selectively attenuates rapidly triggered NMDA-induced neurotoxicity in cortical neurons". Eur. J. Neurosci. 8 (1): 138–43. doi:10.1111/j.1460-9568.1996.tb01174.x. PMID 8713457.
- ^ Bruno V, Battaglia G, Copani A, Giffard RG, Raciti G, Raffaele R, Shinozaki H, Nicoletti F (1995). "Activation of class II or III metabotropic glutamate receptors protects cultured cortical neurons against excitotoxic degeneration". Eur. J. Neurosci. 7 (9): 1906–13. doi:10.1111/j.1460-9568.1995.tb00712.x. PMID 8528465.
- ^ Allen JW, Ivanova SA, Fan L, Espey MG, Basile AS, Faden AI (1999). "Group II metabotropic glutamate receptor activation attenuates traumatic neuronal injury and improves neurological recovery after traumatic brain injury" (abstract). J. Pharmacol. Exp. Ther. 290 (1): 112–20. PMID 10381766. http://jpet.aspetjournals.org/cgi/content/abstract/290/1/112.
- ^ Faden AI, Ivanova SA, Yakovlev AG, Mukhin AG (1997). "Neuroprotective effects of group III mGluR in traumatic neuronal injury". J. Neurotrauma 14 (12): 885–95. doi:10.1089/neu.1997.14.885. PMID 9475370.
- ^ Wang J-Q, Brownell A-L (2007). Development of metabotropic glutamate receptor ligands for neuroimaging. Current Medical Imaging Reviews 3 (3): 186-205. Retrieved on January 20, 2008.
- ^ Siliprandi R, Lipartiti M, Fadda E, Sautter J, Manev H (1992). "Activation of the glutamate metabotropic receptor protects retina against N-methyl-D-aspartate toxicity". Eur. J. Pharmacol. 219 (1): 173–4. doi:10.1016/0014-2999(92)90598-X. PMID 1397046.
- ^ Baskys A, Fang L, Bayazitov I (2005). "Activation of neuroprotective pathways by metabotropic group I glutamate receptors: a potential target for drug discovery?". Ann. N. Y. Acad. Sci. 1053 (1): 55–73. doi:10.1196/annals.1344.006. PMID 16179509.
- ^ Namkoong J, Shin SS, Lee HJ, Marín YE, Wall BA, Goydos JS, Chen S (2007). "Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma". Cancer Res. 67 (5): 2298–305. doi:10.1158/0008-5472.CAN-06-3665. PMID 17332361.
- ^ Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD (2007). "Efficacy and Tolerability of an mGlu2/3 Agonist in the Treatment of Generalized Anxiety Disorder". Neuropsychopharmacology 33 (7): 1603. doi:10.1038/sj.npp.1301531. PMID 17712352.
- ^ Marino MJ, Williams DL, O'Brien JA, Valenti O, McDonald TP, Clements MK, Wang R, DiLella AG, Hess JF, Kinney GG, Conn PJ (2003). "Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson's disease treatment". Proc. Natl. Acad. Sci. U.S.A. 100 (23): 13668–73. doi:10.1073/pnas.1835724100. PMC 263871. PMID 14593202. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=263871.
- ^ Dölen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF (2007). "Correction of fragile X syndrome in mice". Neuron 56 (6): 955–62. doi:10.1016/j.neuron.2007.12.001. PMC 2199268. PMID 18093519. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2199268.
- ^ Dölen G, Carpenter RL, Ocain TD, Bear MF (2010). "Mechanism-based approaches to treating fragile X". Pharmacol Ther 127 (1): 78–93. doi:10.1016/j.pharmthera.2010.02.008. PMID 20303363.
- ^ Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R (2003). "NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development". Psychopharmacology (Berl.) 169 (3-4): 215–33. doi:10.1007/s00213-003-1582-z. PMID 12955285.
- ^ Ford JM, Krystal JH, Mathalon DH (2007). "Neural synchrony in schizophrenia: from networks to new treatments". Schizophr Bull 33 (4): 848–52. doi:10.1093/schbul/sbm062. PMC 2632315. PMID 17567628. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2632315.
- ^ Homayoun H, Jackson ME, Moghaddam B (2005). "Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats". J. Neurophysiol. 93 (4): 1989–2001. doi:10.1152/jn.00875.2004. PMID 15590730.
- ^ Moghaddam B, Adams BW (1998). "Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats". Science 281 (5381): 1349–52. doi:10.1126/science.281.5381.1349. PMID 9721099.
- ^ Krystal JH, Abi-Saab W, Perry E, D'Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, Breier A (2005). "Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects". Psychopharmacology (Berl.) 179 (1): 303–9. doi:10.1007/s00213-004-1982-8. PMID 15309376.
- ^ Aghajanian GK, Marek GJ (2000). "Serotonin model of schizophrenia: emerging role of glutamate mechanisms". Brain Res. Brain Res. Rev. 31 (2-3): 302–12. doi:10.1016/S0165-0173(99)00046-6. PMID 10719157.
- ^ Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD (2007). "Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial". Nat. Med. 13 (9): 1102–7. doi:10.1038/nm1632. PMID 17767166.
- ^ Sladeczek F, Pin JP, Récasens M, Bockaert J, Weiss S (1985). "Glutamate stimulates inositol phosphate formation in striatal neurones". Nature 317 (6039): 717–9. doi:10.1038/317717a0. PMID 2865680.
- ^ Sugiyama H, Ito I, Hirono C (1987). "A new type of glutamate receptor linked to inositol phospholipid metabolism". Nature 325 (6104): 531–3. doi:10.1038/325531a0. PMID 2880300.
- ^ Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S (February 1991). "Sequence and expression of a metabotropic glutamate receptor". Nature 349 (6312): 760–5. doi:10.1038/349760a0. PMID 1847995.
- ^ Dingledine R, Borges K, Bowie D, Traynelis SF (March 1999). "The glutamate receptor ion channels". Pharmacol. Rev. 51 (1): 7–61. PMID 10049997. http://pharmrev.aspetjournals.org/cgi/pmidlookup?view=long&pmid=10049997.
- ^ Wang Y, Small DL, Stanimirovic DB, Morley P, Durkin JP (October 1997). "AMPA receptor-mediated regulation of a Gi-protein in cortical neurons". Nature 389 (6650): 502–4. doi:10.1038/39062. PMID 9333240.
External links
Glutamatergics Ionotropic Agonists: 5-Fluorowillardiine • AMPA • Domoic acid • Quisqualic acid; Positive allosteric modulators: Aniracetam • Cyclothiazide • CX-516 • CX-546 • CX-614 • CX-691 • CX-717 • Diazoxide • HCTZ • IDRA-21 • LY-392,098 • LY-404,187 • LY-451,395 • LY-451,646 • LY-503,430 • Oxiracetam • PEPA • Piracetam • Pramiracetam • S-18986 • Sunifiram • Unifiram
Antagonists: ATPO • Barbiturates • Caroverine • CNQX • DNQX • GYKI-52466 • NBQX • Perampanel • Talampanel • Tezampanel • Topiramate; Negative allosteric modulators: GYKI-53,655Agonists: Glutamate/acite site competitive agonists: Aspartate • Glutamate • Homoquinolinic acid • Ibotenic acid • NMDA • Quinolinic acid • Tetrazolylglycine; Glycine site agonists: ACBD • ACPC • ACPD • Alanine • CCG • Cycloserine • DHPG • Fluoroalanine • Glycine • HA-966 • L-687,414 • Milacemide • Sarcosine • Serine • Tetrazolylglycine; Polyamine site agonists: Acamprosate • Spermidine • Spermine
Antagonists: Competitive antagonists: AP5 (APV) • AP7 • CGP-37849 • CGP-39551 • CGP-39653 • CGP-40116 • CGS-19755 • CPP • LY-233,053 • LY-235,959 • LY-274,614 • MDL-100,453 • Midafotel (d-CPPene) • NPC-12,626 • NPC-17,742 • PBPD • PEAQX • Perzinfotel • PPDA • SDZ-220581 • Selfotel; Noncompetitive antagonists: ARR-15,896 • Caroverine • Dexanabinol • FPL-12495 • FR-115,427 • Hodgkinsine • Magnesium • MDL-27,266 • NPS-1506 • Psychotridine • Zinc; Uncompetitive pore blockers: 2-MDP • 3-MeO-PCP • 8A-PDHQ • Alaproclate • Amantadine • Aptiganel • ARL-12,495 • ARL-15,896-AR • ARL-16,247 • Budipine • Delucemine • Dexoxadrol • Dextrallorphan • Dieticyclidine • Dizocilpine • Endopsychosin • Esketamine • Etoxadrol • Eticyclidine • Gacyclidine • Ibogaine • Indantadol • Ketamine • Ketobemidone • Loperamide • Memantine • Meperidine (Pethidine) • Methadone • Methorphan (Dextromethorphan, Levomethorphan) • Methoxetamine • Milnacipran • Morphanol (Dextrorphan, Levorphanol) • NEFA • Neramexane • Nitrous oxide • Noribogaine • Orphenadrine • PCPr • Phencyclamine • Phencyclidine • Propoxyphene • Remacemide • Rhynchophylline • Riluzole • Rimantadine • Rolicyclidine • Sabeluzole • Tenocyclidine • Tiletamine • Tramadol • Xenon; Glycine site antagonists: ACEA-1021 • ACEA-1328 • ACPC • Carisoprodol • CGP-39653 • CKA • DCKA • Felbamate • Gavestinel • GV-196,771 • Kynurenic acid • L-689,560 • L-701,324 • Lacosamide • Licostinel • LU-73,068 • MDL-105,519 • Meprobamate • MRZ 2/576 • PNQX • ZD-9379; NR2B subunit antagonists: Besonprodil • CO-101,244 (PD-174,494) • CP-101,606 • Eliprodil • Haloperidol • Ifenprodil • Isoxsuprine • Nylidrin • Ro8-4304 • Ro25-6981 • Traxoprodil; Polyamine site antagonists: Arcaine • Co 101676 • Diaminopropane • Acamprosate • Diethylenetriamine • Huperzine A • Putrescine • Ro 25-6981; Unclassified/unsorted antagonists: Chloroform • Diethyl ether • Enflurane • Ethanol (Alcohol) • Halothane • Isoflurane • Methoxyflurane • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • XyleneAgonists: 5-Iodowillardiine • ATPA • Domoic acid • Kainic acid • LY-339,434 • SYM-2081
Antagonists: CNQX • DNQX • LY-382,884 • NBQX • NS102 • Tezampanel • Topiramate • UBP-302; Negative allosteric modulators: NS-3763Metabotropic Agonists: Unselective: ACPD • DHPG • Quisqualic acid; mGlu1-selective: Ro01-6128 • Ro67-4853 • Ro67-7476 • VU-71; mGlu5-selective: ADX-47273 • CDPPB • CHPG • DFB • VU-1545
Antagonists: Unselective: MCPG • NPS-2390; mGlu1-selective: BAY 36-7620 • CPCCOEt • LY-367,385 • LY-456,236; mGlu5-selective: Dipraglurant • DMeOB • Fenobam • LY-344,545 • MPEP • MTEP • SIB-1757 • SIB-1893Agonists: Unselective: L-AP4; mGlu4-selective: PHCCC • VU-001,171 • VU-0155,041; mGlu7-selective: AMN082; mGlu8-selective: DCPG
Antagonists: Unselective: CPPG • MAP4 • MSOP • MPPG • MTPG • UBP-1112; mGlu7-selective: MMPIPTransporter
inhibitorsDHKA • PDC • WAY-213,613vGluTs7-CKA • Evans blueCategories:- G protein coupled receptors
- Protein domains
- Protein families
Wikimedia Foundation. 2010.