- Neurotransmitter receptor
-
A Neurotransmitter receptor is a membrane receptor protein[1] that is activated by a Neurotransmitter.[2] A membrane protein interacts with the lipid bilayer that encloses the cell and a membrane receptor protein interacts with a chemical in the cells external environment, which binds to the cell.[1] Membrane receptor proteins, in neuronal and glial cells, allow cells to communicate with one another through chemical signals.
In postsynaptic cells, neurotransmitter receptors receive signals that trigger an electrical signal, by regulating the activity of ion channels. The binding of neurotransmitters to specific receptors can the membrane potential of a neuron. This can result in a signal that runs along the axon and can be passed along a neural network[1] (see action potential). On presynaptic cells the binding of a neurotransmitter to a specific receptor provides feedback and mediates excessive neurotransmitter release.[3]
There are two types of Neurotransmitter receptors: ligand-gated receptors or ionotropic receptors and G protein-coupled receptors or metabotropic receptors.[2][4] Ligand-gated receptors can be excited by neurotransmitters (ligands) like glutamate and aspartate. These receptors can also be inhibited by neurotransmitters like GABA and glycine. Conversely, G protein-coupled receptors are neither excitatory nor inhibitory. Rather, they modulate the actions of excititory and inhibitory neurotransmitters.[2] Most neurotransmitters receptors are G-protein coupled.[1]
Contents
Ionotropic receptors: Neurotransmitter-Gated Ion Channels
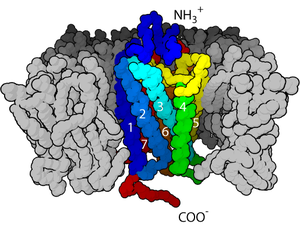
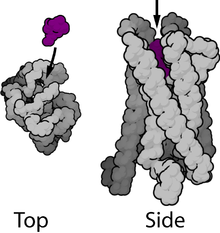
Ligand-gated ion channels (LGICs) are one type of ionotropic receptor or channel-linked receptor. They are a group of transmembrane ion channels that are opened or closed in response to the binding of a chemical messenger (i.e., a ligand),[5] such as a neurotransmitter.[6]
The binding site of endogenous ligands on LGICs protein complexes are normally located on a different portion of the protein (an allosteric binding site) compared to where the ion conduction pore is located. The direct link between ligand binding and opening or closing of the ion channel, which is characteristic of ligand-gated ion channels, is contrasted with the indirect function of metabotropic receptors, which use second messengers. LGICs are also different from voltage-gated ion channels (which open and close depending on membrane potential), and stretch-activated ion channels (which open and close depending on mechanical deformation of the cell membrane).[6][7]
Metabotropic Receptors: G-Protein Coupled Receptors
G protein-coupled receptors (GPCRs), also known as seven-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptor, and G protein-linked receptors (GPLR), comprise a large protein family of transmembrane receptors that sense molecules outside the cell and activate inside signal transduction pathways and, ultimately, cellular responses. G protein-coupled receptors are found only in eukaryotes, including yeast, choanoflagellates,[8] and animals. The ligands that bind and activate these receptors include light-sensitive compounds, odors, pheromones, hormones, and neurotransmitters, and vary in size from small molecules to peptides to large proteins. G protein-coupled receptors are involved in many diseases, and are also the target of approximately 30% of all modern medicinal drugs.[9][10]
There are two principal signal transduction pathways involving the G protein-coupled receptors: the cAMP signal pathway and the Phosphatidylinositol signal pathway.[11] When a ligand binds to the GPCR it causes a conformational change in the GPCR, which allows it to act as a guanine nucleotide exchange factor (GEF). The GPCR can then activate an associated G-protein by exchanging its bound GDP for a GTP. The G-protein's α subunit, together with the bound GTP, can then dissociate from the β and γ subunits to further affect intracellular signaling proteins or target functional proteins directly depending on the α subunit type (Gαs, Gαi/o, Gαq/11, Gα12/13).[12]:1160
Desensitization and Neurotransmitter Concentration
Neurotransmitter receptors are subject to ligand-induced desensitization: That is, they can become unresponsive upon prolonged exposure to their neurotransmitter. Neurotransmitter receptors are present on both postsynaptic neurons and presynaptic neurons with the former being used to receive neurotransmitters and the latter for the purpose of preventing further release of a given neurotransmitter [1]. In addition to being found in neuron cells, neurotransmitter receptors are also found in various immune and muscle tissues [2]. Many neurotransmitter receptors are categorized as a serpentine receptor or G protein-coupled receptor because they span the cell membrane not once, but seven times. Neurotransmitter receptors are known to become unresponsive to the type of neurotransmitter they receive when exposed for extended periods of time. This phenomenon is known as ligand-induced desensitization [3] or downregulation.
Table of neurotransmitters
Neurotransmitters
Transmitter Molecule
Derived From
Site of Synthesis
Acetylcholine [4]
Choline
CNS, parasympathetic nerves
Serotonin [5]
5-Hydroxytryptamine (5-HT)Tryptophan
CNS, chromaffin cells of the gut, enteric cells
GABA [6]
Glutamate
CNS
Glutamate
CNS
Aspartate
CNS
Glycine
spinal cord
Histamine
Histidine
hypothalamus
Epinephrine [7]
synthesis pathway [8]
Tyrosine
adrenal medulla, some CNS cells
Norpinephrine [9]
synthesis pathway [10]
Tyrosine
CNS, sympathetic nerves
Dopamine [11]
synthesis pathway [12]
Tyrosine
CNS
Adenosine
ATP
CNS, periperal nerves
ATP
sympathetic, sensory and enteric nerves
Nitric oxide, NO [13]
Arginine
CNS, gastrointestinal tract
Example neurotransmitter receptors[13]
α1A, α1b, α1c, α1d α2a, α2b, α2c, α2d β1, β2, β3
D1, D2, D3, D4, D5
GABAA, GABAB1a, GABAB1δ, GABAB2, GABAC
NMDA, AMPA kainate, mGluR1, mGluR2, mGluR3, mGluR4, mGluR5, mGluR6, mGluR7
H1, H2, H3
Muscarinic: M1, M2, M3, M4, M5 Nicotinic: muscle, neuronal (α-bungarotoxin-insensitive), neuronal (α-bungarotoxin-sensitive)
μ, δ1, δ2, κ
5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, 5-HT1F, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7
GlycineSee also
- Autoreceptor
- Heteroreceptor
- Receptor desensitization has been previously modeled in the context of a two-state mathematical model (see this link [14])
- Synaptic Transmission
- Neuromuscular transmission
- Catecholamines
- Cholinergic Agonists and Antagonists
Notes and references
- ^ a b c d Levitan, Irwin B.; Leonard K. Kaczmarek (2002). The Neuron (Third pg. 285 ed.). Oxford University Press.
- ^ a b c http://www.brainexplorer.org/neurological_control/neurological_neurotransmitters.shtml
- ^ http://www.rndsystems.com/molecule_group.aspx?g=682&r=9
- ^ http://web.williams.edu/imput/synapse/pages/III.html
- ^ "ligand-gated channel" at Dorland's Medical Dictionary
- ^ a b Purves, Dale, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and Leonard E. White (2008). Neuroscience. 4th ed.. Sinauer Associates. pp. 156–7. ISBN 978-0-87893-697-7.
- ^ Connolly CN, Wafford KA (2004). "The Cys-loop superfamily of ligand-gated ion channels: the impact of receptor structure on function". Biochem. Soc. Trans. 32 (Pt3): 529–34. doi:10.1042/BST0320529. PMID 15157178.
- ^ King N, Hittinger CT, Carroll SB (2003). "Evolution of key cell signaling and adhesion protein families predates animal origins". Science 301 (5631): 361–3. doi:10.1126/science.1083853. PMID 12869759.
- ^ Filmore, David (2004). "It's a GPCR world". Modern Drug Discovery (American Chemical Society) 2004 (November): 24–28. http://pubs.acs.org/subscribe/journals/mdd/v07/i11/html/1104feature_filmore.html.
- ^ Overington JP, Al-Lazikani B, Hopkins AL (December 2006). "How many drug targets are there?". Nat Rev Drug Discov 5 (12): 993–6. doi:10.1038/nrd2199. PMID 17139284.
- ^ Gilman A.G. (1987). "G Proteins: Transducers of Receptor-Generated Signals". Annual Review of Biochemistry 56: 615–649. doi:10.1146/annurev.bi.56.070187.003151. PMID 3113327.
- ^ Wettschureck N, Offermanns S (October 2005). "Mammalian G proteins and their cell type specific functions". Physiol. Rev. 85 (4): 1159–204. doi:10.1152/physrev.00003.2005. PMID 16183910.
- ^ ed. Kebabain, J. W. & Neumeyer, J. L. (1994). "RBI Handbook of Receptor Classification"
External links
- Brain Explorer
- Neurotransmitters Postsynaptic Receptors
- Snyder (2009) Neurotransmitters, Receptors, and Second Messengers Galore in 40 Years. Journal of Neuroscience. 29(41): 12717-12721.
- Snyder and Bennett (1976) Neurotransmitter Receptors in the Brain: Biochemical Identification. Annual Review of Physiology. Vol. 38: 153-175
- Neuroscience for Kids: Neurotransmitters
- NIDA for Teens: Fats on Drugs and Addiction, Brain and Addiction
- Library of Congress Authorities and Vocabularies: Neurotransmitter Receptors
- Neurotransmitter Receptors, Transporters, & Ion Channels
Membrane proteins, receptors: cell surface receptors G protein-coupled receptor Class AEicosanoid receptor (Prostaglandin receptor) · Protease-activated receptor · Neurotransmitter receptor · Purinergic receptor · Biogenic amine receptor · Olfactory receptorClass BClass CClass DPheromone receptorClass EcAMP receptorClass FLigand-gated ion channel Enzyme-linked receptor Other/ungrouped Asialoglycoprotein receptor · Tumor necrosis factor receptor · Immunoglobulin superfamily · N-Acetylglucosamine receptor · Neuropilins · Transferrin receptor · EDAR · Lipoprotein receptor-related proteinsee also cell surface receptor deficiencies
B trdu: iter (nrpl/grfl/cytl/horl), csrc (lgic, enzr, gprc, igsr, intg, nrpr/grfr/cytr), itra (adap, gbpr, mapk), calc, lipd; path (hedp, wntp, tgfp+mapp, notp, jakp, fsap, hipp, tlrp)Categories:- Receptors
Wikimedia Foundation. 2010.