- Membrane protein
-
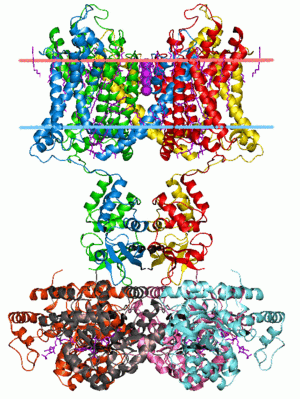 Crystal structure of Potassium channel KvAP. Calculated hydrocarbon boundaries of the lipid bilayer are indicated by red and blue dots.
Crystal structure of Potassium channel KvAP. Calculated hydrocarbon boundaries of the lipid bilayer are indicated by red and blue dots.
A membrane protein is a protein molecule that is attached to, or associated with the membrane of a cell or an organelle. More than half of all proteins interact with membranes.
Contents
Function
Biological membranes consist of a phospholipid bilayer and a variety of proteins that accomplish vital biological functions.
- Structural proteins are attached to microfilaments in the cytoskeleton which ensures stability of the cell.
- Cell adhesion molecules allow cells to identify each other and interact. Such proteins are involved in immune response, for example.
- Membrane enzymes produce a variety of substances essential for cell function.
- Membrane receptor proteins serve as connection between the cell's internal and external environments.
- Transport proteins play an important role in the maintenance of concentrations of ions. These transport proteins come in two forms: carrier proteins and channel proteins.
Main categories
Membrane proteins can be divided into several categories:[1]
- Integral membrane proteins which are permanently bound to the lipid bilayer
- Peripheral membrane proteins that are temporarily associated with lipid bilayer or with integral membrane proteins
- Lipid-anchored proteins bound to lipid bilayer bound through lipidated amino acid residues
In addition, pore-forming toxins and many antibacterial peptides are water-soluble molecules, but undergo a conformational transition upon association with lipid bilayer and become reversibly or irreversibly membrane-associated.
A slightly different classification is to divide all membrane proteins to integral and amphitropic.[2] The amphitropic are proteins that can exist in two alternative states: a water-soluble and a lipid bilayer-bound. The amphitropic protein category includes water-soluble channel-forming polypeptide toxins, which associate irreversibly with membranes, but excludes peripheral proteins that interact with other membrane proteins rather than with lipid bilayer.
Integral membrane proteins
Integral membrane proteins are permanently attached to the membrane. They can be defined as those proteins which require a detergent (such as SDS or Triton X-100) or some other apolar solvent to be displaced. They can be classified according to their relationship with the bilayer:
- Integral polytopic proteins, also known as "transmembrane proteins," are proteins that are permanently attached to the lipid membrane and span across the membrane (at least once). The transmembrane regions of the proteins are either beta-barrels or alpha-helical. The alpha-helical domains are present in all types of biological membranes including outer membranes[disambiguation needed
 ]. The beta-barrels were found only in outer membranes of Gram-negative bacteria, lipid-rich cell walls of a few Gram-positive bacteria, and outer membranes of mitochondria and chloroplasts.
]. The beta-barrels were found only in outer membranes of Gram-negative bacteria, lipid-rich cell walls of a few Gram-positive bacteria, and outer membranes of mitochondria and chloroplasts.
- Integral monotopic proteins are proteins that are permanently attached to the lipid membrane from only one side and do not span across the membrane.
Peripheral membrane proteins
Peripheral membrane proteins are also known as extrinsic proteins, they do not interact with the hydrophobic core of the lipid bilayer. Some peripheral membrane proteins are located at the outer part of the plasma membrane (exoplasmic). They interact with the membrane indirectly by binding to the integral membrane proteins.
Peripheral membrane proteins are temporarily attached either to the lipid bilayer or to integral proteins by a combination of hydrophobic, electrostatic, and other non-covalent interactions. Peripheral proteins dissociate following treatment with a polar reagent, such as a solution with an elevated pH or high salt concentrations.
Integral and peripheral proteins may be post-translationally modified, with added fatty acid or prenyl chains, or GPI (glycosylphosphatidylinositol), which may be anchored in the lipid bilayer.
Polypeptide toxins
Main article: Pore-forming toxinPolypeptide toxins, such as colicins or hemolysins, and certain proteins involved in apoptosis, are sometimes considered a separate category. These proteins are water-soluble but can aggregate and associate irreversibly with the lipid bilayer and form alpha-helical or beta-barrel transmembrane channels.
Intracellular localization
Proteins are specifically targeted to many different types of biological membranes [4]
Membrane Protein Complexes
Membrane Proteins commonly function as complexes. These complexes are vital to cellular function. Understanding how these complexes are assembled, degraded, and their composition are crucial to understanding their function and regulation. Reoccurring in recent literature are the ideas that: membrane protein complexes assemble in an orderly fashion, chaperones aid assembly by preventing unfavorable interactions, and membrane proteins can be interchanged in existing complexes. Membrane protein complexes assemble through the orderly assembly of intermediates. For example, the simple membrane-embedded four subunit complex, cytochrome bo3 of Escherichia coli, is assembled via two intermediate complexes. This suggests a linearly organized assembly pathway. Although interactions between other subunits could lead to the formation of many intermediates, they do not occur. Ordered assembly could be the cell's protection against harmful intermediates. Chaperones interact with membrane proteins guiding their assembly. They aid in preventing the assembly of dead-end and toxic intermediates, as well as unwanted aggregations. Via chaperones assembly can occur through inactive intermediates potentially preventing damaging interactions they could cause. Membrane protein complexes are not fixed entities. Though a process called dynamic exchange, membrane proteins are exchanged in and out of exsitisting protein complexes. This has its implications as a repair mechanism and in regulation. [5]
Assembly of Membrane Protein
There are two different ways a membrane protein could undergoes during construction are Constitutive membrane protein and non-constitutive membrane protein.
Constitutive Membrane Protein- The messengerRNA attaches to the translocon which is located to the cell membrane. The mRNA is translated into the translocon transmembrane tunnel. After the synthesis of protein the mRNA is released which closes the translocon and the protein is released in the bilayer membrane. When the protein is left in the membrane bilayer more protein folding occurs creating its final 3D structure (White).
Non-constitutive Membrane Protein- Examples of non-constitutive membrane proteins are toxins and antimicrobialpeptides. These membrane proteins inserts into specific target membranes by physicochemical processes. It inserts usually before, during, or after oligomerization into the target membrane (White).
Membrane Protein Structures
In membrane proteins the two known structural classes of membrane proteins are alpha-helical bundle and beta-barrel porin. The portion of the membrane proteins that are attached to the lipid bilayer are consisting of hydophobic amino acids only. This is done so that the peptide bonds' carbonyl and amine will react with each other instead of the hydrophobic surrounding. The portion of the protein that is not touching the lipid bilayer and is protruding out of the cell membrane are usually hydrophilic amino acids.
The structures of membrane proteins are stabilized by weak interactions and influenced by additional interactions with the solubilizing environment. The influence of the environment on membrane protein structures is especially significant. Despite the significant functional importance of membrane proteins, the structural biology has been particularly challenging as shown by the low number of membrane protein structures determined. Integral membrane proteins are present in a heterogeneous environment that poses major obstacles for existing structural methodologies.
Many of the successful membrane protein structures are characterized by X-ray crystallography and are very large structures in which the interactions with the membrane mimetic environments can be anticipated to be small in comparison to those within the protein structures. The small domains are particularly sensitive to the influence of membrane mimetic environments, potentially leading to non-native structures. Fortunately, there are many sample preparation conditions that can be chosen for crystallization and for solution NMR. All membrane protein structural biology should be subjected to careful scrutiny; through a combination of structural methodologies it should be possible to achieve an understanding of the native functional state for membrane protein structures.[8]
See also
- Integral membrane proteins
- Transmembrane proteins
- Peripheral membrane proteins
- Ion pump (biology)
- Carrier protein
- Ion channel
- Receptor (biochemistry) (including G protein-coupled receptor)
- List of MeSH codes (D12.776)
References
White, Stephen. “General Principle of Membrane Protein Folding and Stability.” Stephen White Laboratory Homepage. 10 Nov. 2009. web.
- ^ Gerald Karp (2009). Cell and Molecular Biology: Concepts and Experiments. John Wiley and Sons. pp. 128–. ISBN 9780470483374. http://books.google.com/books?id=arRGYE0GxRQC&pg=PA128. Retrieved 13 November 2010.
- ^ Johnson JE, Cornell RB (1999). "Amphitropic proteins: regulation by reversible membrane interactions (review)". Mol. Membr. Biol. 16 (3): 217–235. doi:10.1080/096876899294544. PMID 10503244.
- ^ Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000.
- ^ Classification of membrane proteins with known 3D structure to different membrane types
- ^ Daley, Daniel. 2008,"The Assembly of Membrane Proteins into Complexes", Current Opinion in Structural Biology, 18:420-424.
- ^ White, Stephen. “General Principle of Membrane Protein Folding and Stability.” Stephen White Laboratory Homepage. 10 Nov. 2009. web.
- ^ White, Stephen. “General Principle of Membrane Protein Folding and Stability.” Stephen White Laboratory Homepage. 10 Nov. 2009. web.
- ^ Cross, Timothy, Mukesh Sharma, Myunggi Yi, Huan-Xiang Zhou (2010). "Influence of Solubilizing Environments on Membrane Protein Structures"
External links
- TCDB - Transporter Classification database
- Orientations of Proteins in Membranes (OPM) database 3D structures of integral and peripheral membrane proteins arranged in the lipid bilayer
- The Protein Data Bank of Transmembrane Proteins 3D models of all transmembrane proteins currently in PDB. Approximate positions of membrane boundary planes were calculated for each PDB entry.
- List of transmembrane proteins of known 3D structure
- TransportDB Genomics-oriented database of transporters from TIGR
- Membrane PDB Database of 3D structures of integral membrane proteins and hydrophobic peptides with an emphasis on crystallization conditions
- Membrane targeting domains (MeTaDoR)
- Antimicrobial Peptide Database
- MeSH Membrane+proteins
- The Human Membrane Proteome - A comprehensive article covering the transmembrane protein component of the human proteome
Processes Structures Types List of types of proteins · List of proteins · Membrane protein · Globular protein (Globulin, Albumin) · Fibrous proteinbiochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/i
B proteins: BY STRUCTURE: membrane, globular (en, ca, an), fibrousStructures of the cell membrane Membrane lipids Membrane protein locations Membrane glycoproteins, Integral membrane proteins/transmembrane protein, Peripheral membrane protein/Lipid-anchored proteinOther B memb: cead, trns (1A, 1C, 1F, 2A, 3A1, 3A2-3, 3D), othr Categories:- WikiProject Molecular and Cellular Biology articles
- Membrane biology
- Membrane proteins
Wikimedia Foundation. 2010.
