- Choanoflagellate
-
Choanoflagellates 
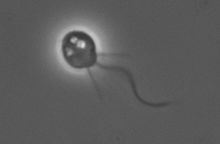
Scientific classification Domain: Eukarya (unranked) Opisthokonta (unranked) Choanozoa or Holozoa (unranked) Filozoa Class: Choanoflagellatea The choanoflagellates are a group of free-living unicellular and colonial flagellate eukaryotes considered to be the closest living relatives of the animals. As the name suggests, choanoflagellates (collared flagellates) have a distinctive cell morphology characterized by an ovoid or spherical cell body 3-10 µm in diameter with a single apical flagellum surrounded by a collar of 30-40 microvilli (see figure). Movement of the flagellum creates water currents that can propel free-swimming choanoflagellates through the water column and trap bacteria and detritus against the collar of microvilli where these foodstuffs are engulfed. This feeding provides a critical link within the global carbon cycle, linking trophic levels. In addition to their critical ecological roles, choanoflagellates are of particular interest to evolutionary biologists studying the origins of multicellularity in animals. As the closest living relatives of animals, choanoflagellates serve as a useful model for reconstructions of the last unicellular ancestor of animals.
Contents
Appearance and growth
Each choanoflagellate has a single flagellum, surrounded by a ring of actin-filled protrusions called microvilli, forming a cylindrical or conical collar (choanos in Greek). Movement of the flagellum draws water through the collar, and bacteria and detritus are captured by the microvilli and ingested.[1] Water currents generated by the flagellum also push free-swimming cells along, as in animal sperm. In contrast, most other flagellates are pulled by their flagella.
In addition to the single apical flagellum surrounded by actin-filled microvilli that characterizes choanoflagellates, the internal organization of organelles in the cytoplasm is constant.[2] A flagellar basal body sits at the base of the apical flagellum, and a second, non-flagellar basal body rests at a right angle to the flagellar base. The nucleus occupies an apical-to-central position in the cell, and food vacuoles are positioned in the basal region of the cytoplasm.[2][3] Additionally, the cell body of many choanoflagellates is surrounded by a distinguishing extracellular matrix or periplast. These cell coverings vary greatly in structure and composition and are used by taxonomists for classification purposes. Many choanoflagellates build complex basket-shaped "houses" called lorica, from several silica strips cemented together.[2] The functional significance of the periplast is unknown, but in sessile organisms, it is thought to aid in attachment to the substrate. In planktonic organisms, there is speculation that the periplast increases drag, thereby counteracting the force generated by the flagellum and increasing feeding efficiency.[4] Choanoflagellates are either free-swimming in the water column or sessile, adhering to the substrate directly or through either the periplast or a thin pedicel.[5] Although choanoflagellates are thought to be strictly free-living and heterotrophic, a number of choanoflagellate relatives such as members of Ichthyosporea or Mesomycetozoa follow a parasitic or pathogenic lifestyle.[6] The life histories of choanoflagellates are poorly understood. Many species are thought to be solitary; however coloniality seems to have arisen independently several times within the group and colonial species retain a solitary stage.[5]
Choanoflagellates grow vegetatively, with many species undergoing longitudinal fission;[3] however, the reproductive life cycle of choanoflagellates remains to be elucidated. Currently, it is unclear whether there is a sexual phase to the choanoflagellate life cycle, however the discovery of both retrotransposons and key genes involved in meiosis [7] suggests they are cryptically sexual. Interestingly, some choanoflagellates can undergo encystment, which involves the retraction of the flagellum and collar and encasement in an electron dense fibrillar wall. Upon transfer to fresh media excystment occurs, though it remains to be directly observed.[8] Further examination of the choanoflagellate life cycle will be informative about mechanisms of colony formation and attributes present before the evolution of animal multicellularity.
Colonial behaviour
A number of species such as those in the genus Proterospongia form simple colonies,[1] planktonic clumps that resemble a miniature cluster of grapes in which each cell in the colony is flagellated or clusters of cells on a single stalk.[2][9]
Ecology
There are over 125 extant species of choanoflagellates[1] distributed globally in marine, brackish and freshwater environments from the Arctic to the tropics, occupying both pelagic and benthic zones. Although most sampling of choanoflagellates has occurred between 0 m and 25 m, they have been recovered from as deep as 300 m in open water[10] and 100 m under Antarctic ice sheets.[11] Many species are hypothesized to be cosmopolitan on a global scale [e.g., Diaphanoeca grandis has been reported from North America, Europe and Australia (OBIS)], while other species are reported to have restricted regional distributions.[12] Co-distributed choanoflagellate species can occupy quite different microenvironments, but in general, the factors that influence the distribution and dispersion of choanoflagellates remain to be elucidated.
The choanoflagellates feed on bacteria and link otherwise inaccessible forms of carbon to organisms higher in the trophic chain.[13] Even today they are important in the carbon cycle and microbial food web.[1]
Classification
Phylogenetic relationships
Recent molecular phylogenetic reconstruction of the internal relationships of choanoflagellates and allows the polarization character evolution within the clade. Large fragments of the nuclear SSU and LSU ribosomal RNA, alpha tubulin, and heat-shock protein 90 coding genes were used to resolve the internal relationships and character polarity within choanoflagellates.[9] Each of the four genes showed similar results independently and analysis of the combined data set (concatenated) along with sequences from other closely related species (animals and fungi) demonstrate that choanoflagellates are strongly supported as monophyletic and the closest known living relative of animals. The choanoflagellate tree divides into three well supported clades.[9] Clade 1 and Clade 2 each consist of a combination of species traditionally attributed to the Codonosigidae and Salpingoecidae while Clade 3 comprises species from the group taxonomically classified as Acanthoecidae.[9] Previously, Choanoflagellida was divided into these three families based upon the composition and structure of their periplast: Codonosigidae, Salpingoecidae and Acanthoecidae. Members of the family Codonosigidae appear to lack a periplast when examined by light microscopy, but may have a fine outer coat visible only by electron microscopy. The family Salpingoecidae consists of species whose cells are encased in a firm theca that is visible by both light and electron microscopy. The theca is a secreted covering predominately composed of cellulose or other polysaccharides (Adl, et al., 2005). The third family of choanoflagellates, the Acanthoecidae, contains species whose cells rest in a basket-like lorica composed of siliceous ribs or "costae."[2][4] The mapping of character traits on to this phylogeny indicates that the last common ancestor of choanoflagellates was a marine organisms with a differentiated life cycle with sedentary and motile stages.[9]
Relationship of choanoflagellates to metazoans
Dujardin, a French biologist interested in protozoan evolution, recorded the morphological similarities of choanoflagellates and sponge choanocytes and proposed the possibility of a close relationship as early as 1841.[4] Over the past decade, this hypothesized relationship between choanoflagellates and animals has been upheld by independent analyses of multiple unlinked sequences: 18S rDNA, nuclear protein-coding genes, and mitochondrial genomes (Steenkamp, et al., 2006; Burger, et al., 2003;[6] Wainright, et al., 1993). Importantly, comparisons of mitochondrial genome sequences from a choanoflagellate and three sponges confirm the placement of choanoflagellates as an outgroup to Metazoa and negate the possibility that choanoflagellates evolved from metazoans (Lavrov, et al., 2005). Finally, recent studies of genes expressed in choanoflagellates have revealed that choanoflagellates synthesize homologues of metazoan cell signaling and adhesion genes.[14] (King, 2003) Genome sequencing shows that among living organisms, the choanoflagellates are most closely related to animals.[1] Because choanoflagellates and metazoans are closely related, comparisons between the two groups promise to provide insights into the biology of their last common ancestor and the earliest events in metazoan evolution. The choanocytes (also known as "collared cells") of sponges (considered the most basal metazoa) have the same basic structure as choanoflagellates. Collared cells are found in other animal groups, such as ribbon worms,[15] suggesting this was the morphology of their last common ancestor. The last common ancestor of animals and choanoflagellates was unicellular, perhaps forming simple colonies; in contrast, the last common ancestor of all eumetazoan animals was a multicellular organism, with differentiated tissues, a definite "body plan", and embryonic development (including gastrulation).[1] The timing of the splitting of these lineages is difficult to constrain, but was probably in the late Precambrian, >600 million years ago.[1]
Monosiga brevicollis genome
The genome of Monosiga brevicollis, with 41.6 million base pairs,[1] is similar in size to filamentous fungi and other free-living unicellular eukaryotes, but far smaller than that of typical animals.[1] Recently, a phylogenomic study revealed that several algal genes are present in the genome of Monosiga brevicollis. This could be due to the fact that in early evolutionary history, Choanoflagellates consumed algae as food through phagocytosis.[16]
References
- ^ a b c d e f g h i King, N.; Westbrook, M.J.; Young, S.L.; Kuo, A.; Abedin, M.; Chapman, J.; Fairclough, S.; Hellsten, U.; Isogai, Y.; Letunic, I.; Others, (2008). "The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans". Nature 451 (7180): 783–8. doi:10.1038/nature06617. PMC 2562698. PMID 18273011. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2562698.
- ^ a b c d e Leadbeater, B.S.C.; Thomsen, H. (2000). "Order Choanoflagellida". An Illustrated Guide to the Protozoa, Second Edition. Lawrence : Society of Protozoologists 451: 14–38.
- ^ a b Karpov S.; Leadbeater, B.S.C. (1998). "Cytoskeleton structure and composition in choanoflagellates". Journal of Eukaryotic Microbiology 45 (3): 361–367. doi:10.1111/j.1550-7408.1998.tb04550.x.
- ^ a b c Leadbeater, B.S.C.; Kelly, M. (2001). "Evolution of animals choanoflagellates and sponges". Water and Atmosphere Online 9 (2): 9–11.
- ^ a b Leadbeater, B.S.C. (1983). "Life-History and Ultrastructure of a New Marine Species of Proterospongia (Choanoflagellida)". J. Mar. Biol. Ass. U.K. 63 (63): 135–160. doi:10.1017/S0025315400049857.
- ^ a b Mendoza L.; Taylor, J. and Ajello, L. (2002). "The class Mesomycetozoea: a heterogeneous group of microorganisms at the animal-fungal boundary". Ann. Rev. Microbiol. 56: 315–44. doi:10.1146/annurev.micro.56.012302.160950. PMID 12142489.
- ^ Carr M.; Leadbeater B., and Baldauf, S. (2002). "Conserved Meiotic Genes Point to Sex in the Choanoflagellates". J. Eukaryot. Microbiol. 57 (1): 56–62. doi:10.1111/j.1550-7408.2009.00450.x.
- ^ Leadbeater, B.S.C.; Karpov, S. (2000). "Cyst Formation in a Freshwater Strain of the Choanoflagellate Desmarella moniliformis Kent". J. Eukaryot. Microbiol. 47 (5): 433–439. doi:10.1111/j.1550-7408.2000.tb00071.x. PMID 11001139.
- ^ a b c d e Carr, M.; Leadbeater, B. S. C.; Hassan, R.; Nelson, M.; Baldauf, S. L. (2008). "Molecular phylogeny of choanoflagellates, the sister group to Metazoa". PNAS 105 (43): 16641–16646. doi:10.1073/pnas.0801667105. PMC 2575473. PMID 18922774. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2575473.
- ^ Thomsen, H. (1982). Planktonic choanoflagellates from Disko Bugt, West Greenland, with a survey of the marine nanoplankton of the area. Meddelelser om Gronland, Bioscience. 8. pp. 3–63. ISBN 978-87-635-1149-0.
- ^ Buck, K.; Garrison, D (1988). "Distribution and abundance of choanoflagellates (Acanthoecidae) across the ice-edge zone in the Weddell Sea, Antarctica". Mar. Biol. 98 (2): 263–269. doi:10.1007/BF00391204. http://www.springerlink.com/index/N141343025V6736T.pdf.
- ^ Thomsen, H.; Buck, K. and Chavez, F. (1991). "Choanoflagellates of the central California waters: Taxonomy, morphology and species assemblages". Ophelia 33: 131–164.
- ^ Butterfield, N.J. (1 April 1997). "Plankton ecology and the Proterozoic-Phanerozoic transition". Paleobiology 23 (2): 247–262. http://paleobiol.geoscienceworld.org/cgi/content/abstract/23/2/247.
- ^ King, N.; Carroll, S.B. (2001). "A receptor tyrosine kinase from choanoflagellates: Molecular insights into early animal evolution". PNAS 98 (26): 15032–15037. doi:10.1073/pnas.261477698. PMC 64978. PMID 11752452. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=64978.
- ^ Cantell, Carl-Erik; Franz�n, �ke; Sensenbaugh, Terry (1982). "Ultrastructure of multiciliated collar cells in the pilidium larva of Lineus bilineatus (Nemertini)". Zoomorphology 101 (1): 1–15. doi:10.1007/BF00312027.
- ^ Sun, Guiling; Arjun Ishwar, Zefeng Yang, Jinling Huang (July 13, 2010). "Algal genes in the closest relatives of animals". Molecular Biology and Evolution 27 (12): 2879–89. doi:10.1093/molbev/msq175. PMID 20627874. http://mbe.oxfordjournals.org/content/27/12/2879.abstract.
External links
- ChoanoWiki a collaborative resource maintained by the Choanoflagellate research community.
- Tree of Life Webpage for Choanoflagellates
- Monosiga brevicollis genome browser
- Choanobase, the Choanoflagellate genetic library, developed and maintained by the Nicole King laboratory at the University of California, Berkeley
Eukaryota Bikonta AH/SARAHSARHalvariaHeterokont ("S")Unikonta Apusomonadida (Apusomonas, Amastigomonas) · Ancyromonadida (Ancyromonas) · Hemimastigida (Hemimastix, Spironema, Stereonema)HolozoaFilozoaFilastereaChoanoflagellateaCategories:- Protista
- Flagellates
Wikimedia Foundation. 2010.



