- Cyanotoxin
-
Green scum produced by and containing cyanobacteria, washed up on a rock in California during an algal bloom

Cyanotoxins are toxins produced by bacteria called cyanobacteria (also known as blue-green algae). Cyanobacteria are found almost everywhere, but particularly in lakes and in the ocean where, under certain conditions, they reproduce exponentially to form blooms. Blooming cyanobacteria can produce cyanotoxins in such concentrations that they poison and even kill animals and humans. Cyanotoxins can also accumulate in other animals such as fish and shellfish, and cause poisonings such as shellfish poisoning.
Among cyanotoxins are some of the most powerful natural poisons known, including poisons which can cause rapid death by respiratory failure.[1] The toxins include potent neurotoxins, hepatotoxins, cytotoxins, and endotoxins. Recreational exposure to cyanobacteria can result in gastro-intestinal and hayfever symptoms or pruritic skin rashes.[2] There is some evidence that significant exposure to high levels of some species of cyanobacteria causes Lou Gehrig's disease.[3][4][5] There is also an interest in the military potential of biological neurotoxins such as cyanotoxins, which "have gained increasing significance as potential candidates for weaponization."[6]
The first published report that blue-green algae or cyanobacteria could have lethal effects appeared in Nature in 1878. George Francis described the algal bloom he observed in the estuary of the Murray River in Australia, as "a thick scum like green oil paint, some two to six inches thick." Wildlife which drank the water died rapidly and terribly.[7] Most reported incidents of poisoning by microalgal toxins have occurred in freshwater environments, and they are becoming more common and widespread. For example, thousands of ducks and geese died drinking contaminated water in the midwestern United States.[8] In 2010, for the first time, marine mammals were reported to have died from ingesting cyanotoxins.[9]
Contents
Cyanobacteria
Main article: CyanobacteriaCyanotoxins are produced by cyanobacteria, a phylum of bacteria that obtain their energy through photosynthesis. The prefix cyan comes from the Greek κύανoς meaning "a dark blue substance",[10] and usually indicates any of a number of colours in the blue/green range of the spectrum. Cyanobacteria are commonly referred to as blue-green algae. Traditionally they were thought of as a form of algae, and were introduced as such in older textbooks. However modern sources tend to regard this as outdated;[11] they are now considered to be more closely related to bacteria,[12] and the term for true algae is restricted to eukaryotic organisms.[13] Like true algae, cyanobacteria are photosynthetic and contain photosynthetic pigments, which is why they are usually green or blue.
Cyanobacteria are found almost everywhere; in oceans, lakes and rivers as well as on land. They flourish in Arctic and Antarctic lakes,[14] hotsprings[15] and wastewater treatments plants.[16] They even inhabit the fur of polar bears, to which they impart a greenish tinge.[17] Cyanobacteria produce potent toxins, but they also produce helpful bioactive compounds, including substances with antitumour, antiviral, anticancer, antibiotic and antifungal activity, UV protectants and specific inhibitors of enzymes.[18][19]
Harmful algal blooms
 Dense bloom of cyanobacteria on the Potomac River estuary. These blooms can be toxic.
Dense bloom of cyanobacteria on the Potomac River estuary. These blooms can be toxic.
Cyanotoxins are often implicated in what are commonly called red tides or harmful algal blooms. Lakes and oceans contain many single-celled organisms called phytoplankton. Under certain conditions, particularly when nutrient concentrations are high, these organisms reproduce exponentially. The resulting dense swarm of phytoplankton is called an algal bloom; these can cover hundreds of square kilometres and can be easily seen in satellite images. Individual phytoplankton rarely live more than a few days, but blooms can last weeks.[20][21]
Generally these blooms are harmless, but if not they are called harmful algal blooms, or HABs. HABs can contain toxins or pathogens which result in fish kill and can also be fatal to humans.[21] In marine environments, HABs are mostly caused by dinoflagellates,[22] though species of other algae taxa can also cause HABs (diatoms, flagellates, haptophytes and raphidophytes).[23] Marine dinoflagellate species are often toxic, but freshwater species are not known to be toxic. Neither are diatoms known to be toxic, at least to humans.[24]
Cyanobacteria also commonly produce blooms and HABs.[22] Cyanobacteria species are often toxic, and in freshwater ecosystems are the most common cause of eutrophication. Their blooms can look like foam, scum or mats or like paint floating on the surface of the water, but they are not always visible. Nor are the blooms always green; they can be blue, and some cyanobacteria species are coloured brownish-red. The water can become malodorous when the cyanobacterial in the bloom die.[21]
Strong cyanobacterial blooms reduce visibility to one or two centimetres. Species which do not need to see to migrate in the water column (such as the cyanobacteria themselves) survive, but species which need to see to find food and partners are compromised. During the day blooming cyanobacteria saturate the water with oxygen. At night respiring aquatic organisms can deplete the oxygen to the point where sensitive species, such as certain fish, die. This is more likely to happen near the sea floor or a thermocline. Water acidity also cycles daily during a bloom, with the pH reaching 9 or more during the day and dropping to low values at night, further stressing the ecosystem. In addition, many cyanobacteria species produce potent cyanotoxins which concentrate during a bloom to the point where they become lethal to nearby aquatic organisms and any other animals in direct contact with the bloom, including birds, livestock, domestic animals and sometimes humans.[24]
In 1991 a harmful cyanobacterial bloom affected 1000 km of the Darling-Barwon River in Australia[25] at an economic cost of $10M AUD.[26]
Chemical structure
The chemical structure of cyanotoxins falls into three broad groups: cyclic peptides, alkaloids and lipopolysaccharides.[27]
Chemical structure of cyanotoxins[27] Structure Cyanotoxin Primary target organ in mammals Cyanobacteria genera Cyclic peptides Microcystins Liver Microcystis, Anabaena, Planktothrix (Oscillatoria), Nostoc, Hapalosiphon, Anabaenopsis Nodularins Liver Nodularia Alkaloids Anatoxin-a Nerve synapse Anabaena, Planktothrix (Oscillatoria), Aphanizomenon Anatoxin-a(S) Nerve synapse Anabaena Aplysiatoxins Skin Lyngbya, Schizothrix, Planktothrix (Oscillatoria) Cylindrospermopsins Liver Cylindrospermopsis, Aphanizomenon, Umezakia Lyngbyatoxin-a Skin, gastro-intestinal tract Lyngbya Saxitoxins Nerve axons Anabaena, Aphanizomenon, Lyngbya, Cylindrospermopsis Lipopolysaccharides Potential irritant; affects any exposed tissue All Most cyanotoxins have a number of variants (analogues). Altogether over 84 cyanotoxins are known although only a small number have been well studied.[19]
Cyclic peptides
A peptide is a short polymer of amino acids linked by peptide bonds. They have the same chemical structure as proteins, except they are shorter. In a cyclic peptide the links link back to the start to form a stable circular chain. In mammals this stability makes them resistant to the process of digestion and they can bioaccumulate in the liver. Of all the cyanotoxins, the cyclic peptides are of most concern to human health. The microcystins and nodularins poison the liver, and exposure to high doses can cause death. Exposure to low doses in drinking water over a long period of time may promote liver and other tumours.[27]
Microcystins
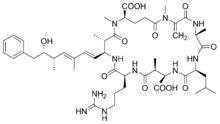
As with other cyanotoxins, microcystins were named after the first organism discovered to produce them, Microcystis aeruginosa. However it was later found other cyanobacterial genera also produced them.[27] There are about 60 known variants of microcystin, and several of these can be produced during a bloom. The most reported variant is microcystin-LR, possible because the earliest commercially available chemical standard analysis was for microcystin-LR.[27]
Blooms containing microcystin are a problem worldwide in freshwater ecosystems.[28] Microcystins are cyclic peptides and can be very toxic for plants and animals including humans. They bioaccumulate in the liver of fish, in the hepatopancreas of mussels, and in zooplankton. They are hepatotoxic and can cause serious damage to the liver in humans.[27] In this way they are similar to the nodularins (below), and together the microcystins and nodularins account for most of the toxic cyanobacterial blooms in fresh and brackish waters.[19] In 2010, a number of sea otters were reported as having been poisoned by microcystin. Marine bivalves were the likely source of hepatotoxic shellfish poisoning. This is the first confirmed example of mammals in a marine environment dying from ingesting a cyanotoxin.[9]
Nodularins
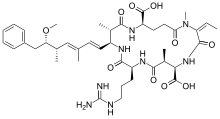
The first nodularin variant to be identified was nodularin-R, produced by the cyanobacterium Nodularia spumigena.[29] This cyanobacterium blooms in water bodies throughout the world. In the Baltic Sea, marine blooms of Nodularia spumigena are among some of the largest cyanobacterial mass events in the world.[30]
Globally, the most common toxins present in cyanobacterial blooms in fresh and brackish waters are the cyclic peptide toxins of the nodularin family. Like the microcystin family (above), nodularins are potent hepatotoxins and can cause serious damage to the liver. They present health risks for wild and domestic animals as well as humans, and in many areas pose major challenges for the provision of safe drinking water.[19]
Alkaloids
Alkaloids are a group of naturally occurring chemical compounds which mostly contain basic nitrogen atoms. They are produced by a large variety of organisms, including cyanobacteria, and are part of the group of natural products, also called secondary metabolites. Alkaloids act on diverse metabolic systems in humans and other animals, often with psychotropic or toxic effects. Almost uniformly, they are bitter tasting.[31]
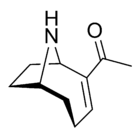
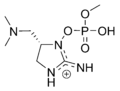
Anatoxin-a
Investigations into anatoxin-a, also known as "Very Fast Death Factor", began in 1961 following the deaths of cows that drank from a lake containing an algal bloom in Saskatchewan, Canada.[32][33] The toxin is produced by at least four different genera of cyanobacteria and has been reported in North America, Europe, Africa, Asia, and New Zealand.
Toxic effects from anatoxin-a progress very rapidly because it acts directly on the nerve cells (neurons) as a neurotoxin. The progressive symptoms of anatoxin-a exposure are loss of coordination, twitching, convulsions and rapid death by respiratory paralysis. The nerve tissues which communicate with muscles contain a receptor called the nicotinic acetylcholine receptor. Stimulation of these receptors causes a muscular contraction. The anatoxin-a molecule is shaped so it fits this receptor, and in this way it mimics the natural neurotransmitter normally used by the receptor, acetylcholine. Once it has triggered a contraction, anatoxin-a does not allow the neurons to return to their resting state, because it is not degraded by cholinesterase which normally performs this function. As a result the muscle cells contract permanently, the communication between the brain and the muscles is disrupted and breathing stops.[34][35]
 External videos
External videos
Very Fast Death Factor
University of NottinghamWhen it was first discovered, the toxin was called the Very Fast Death Factor (VFDF) because when it was injected into the body cavity of mice it induced tremors, paralysis and death within a few minutes. In 1977, the structure of VFDF was determined as a secondary, bicyclic amine alkaloid, and it was renamed anatoxin-a.[36][37] Structurally, it is similar to cocaine.[38] There is continued interest in anatoxin-a because of the dangers it presents to recreational and drinking waters, and because it is a particularly useful molecule for investigating acetylcholine receptors in the nervous system.[1] The deadliness of the toxin means that it has a high military potential as a toxin weapon.[6]
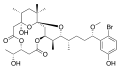
Cylindrospermopsins
Cylindrospermopsin (abbreviated to CYN or CYL) was first discovered after an outbreak of a mystery disease on Palm Island in Australia.[39] The outbreak was traced back to a bloom of Cylindrospermopsis raciborskii in the local drinking water supply, and the toxin was subsequently identified. Analysis of the toxin led to a proposed chemical structure in 1992, which was revised after synthesis was achieved in 2000. Several variants of cylindrospermopsin, both toxic and non-toxic, have been isolated or synthesised.[40]
Cylindrospermopsin is toxic to liver and kidney tissue and is thought to inhibit protein synthesis and to covalently modify DNA and/or RNA. There is concern about the way cylindrospermopsin bioaccumulates in freshwater organisms.[41] Toxic blooms of genera which produce cylindrospermopsin are most commonly found in tropical, subtropical and arid zone water bodies, and have recently been found in Australia, Europe, Israel, Japan and the USA.[27]
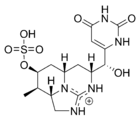
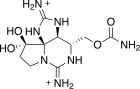
Saxitoxins
Saxitoxin (STX) is one of the most potent natural neurotoxins known. The term saxitoxin originates from the species name of the butter clam (Saxidomus giganteus) whereby it was first recognized. Saxitoxin is produced by the cyanobacteria Anabaena spp., some Aphanizomenon spp., Cylindrospermopsis sp., Lyngbya sp. and Planktothrix sp.).[42] Puffer fish and some marine dinoflagellates also produce saxitoxin.[43][44] Saxitoxins bioaccumulate in shellfish and certain finfish. Ingestion of saxitoxin, usually through shellfish contaminated by toxic algal blooms, can result in paralytic shellfish poisoning.[19]
Saxitoxin has been used in molecular biology to establish the function of the sodium channel. It acts on the voltage-gated sodium channels of nerve cells, preventing normal cellular function and leading to paralysis. The blocking of neuronal sodium channels which occurs in paralytic shellfish poisoning produces a flaccid paralysis that leaves its victim calm and conscious through the progression of symptoms. Death often occurs from respiratory failure.[45] Saxitoxin was originally isolated and described by the United States military, who assigned it the chemical weapon designation "TZ". Saxitoxin is listed in schedule 1 of the Chemical Weapons Convention.[46] According to the book Spycraft, U-2 spyplane pilots were provided with needles containing saxitoxin to be used for suicide in the event escape was impossible.[47]
Lipopolysaccharides
Lipopolysaccharides are present in all cyanobacteria. Though not as potent as other cyanotoxins, some researchers have claimed that all lipopolysaccharides in cyanobacteria can irritate the skin, while other researchers doubt the toxic effects are that generalized.[21][48]
Gallery
Other cyanotoxins:
See also
- beta-Methylamino-L-alanine
- Toxin
- Microbial toxins
- Microbial mats
Notes
- ^ a b Stewart I, Seawright AA, Shaw GR (2008). "Cyanobacterial poisoning in livestock, wild mammals and birds – an overview" (PDF). Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Advances in Experimental Medicine and Biology 619: 613–637. doi:10.1007/978-0-387-75865-7_28. ISBN 978-0-387-75864-0. http://www.epa.gov/cyano_habs_symposium/monograph/Ch28.pdf.
- ^ Stewart I, Webb PM, Schluter PJ, Shaw GR (2006). "Recreational and occupational field exposure to freshwater cyanobacteria – a review of anecdotal and case reports, epidemiological studies and the challenges for epidemiologic assessment". Environmental Health 5 (1): 6. doi:10.1186/1476-069X-5-6. http://www.ehjournal.net/content/5/1/6.
- ^ Cyanobacteria, their toxins and health risks
- ^ Blue-Green Algae (Cyanobacteria) and their Toxins
- ^ Harmful Bloom in Lake Atitlán, Guatemala from NASA Earth Observatory, retrieved on 9 January 2010.
- ^ a b Dixit A, Dhaked RK, Alam SI, Singh L (2005). "Military potential of biological neurotoxins". Informa Healthcare 24 (2): 175–207. doi:10.1081/TXR-200057850.
- ^ Francis G (1878). "Poisonous Australian Lake". Nature 18 (444): 11–12. doi:10.1038/018011d0. http://www.nature.com/nature/journal/v18/n444/abs/018011d0.html.
- ^ Anatoxin Neil Edwards, University of Sussex at Brighton. Updated 1 September 1999. Retrieved 19 January 2011.
- ^ a b Miller MA, Kudela RM, Mekebri A, Crane D, Oates SC et al. (2010). Thompson, Ross. ed. "Evidence for a Novel Marine Harmful Algal Bloom: Cyanotoxin (Microcystin) Transfer from Land to Sea Otters". PLoS ONE 5 (9): e12576. doi:10.1371/journal.pone.0012576. PMC 2936937. PMID 20844747. http://dx.plos.org/10.1371/journal.pone.0012576.
- ^ κύανος, Henry George Liddell, Robert Scott, A Greek-English Lexicon, on Perseus
- ^ Nabors, Murray W. (2004). Introduction to Botany. San Francisco, CA: Pearson Education, Inc. ISBN 0-8053-4416-0.
- ^ Ed. Guiry, M.D., John, D.M., Rindi, F and McCarthy, T.K. 2007. New Survey of Clare Island Volume 6: The Freshwater and Terrestrial Algae. Royal Irish Academy. isbn 13: 978-1-904890-31-7
- ^ Allaby, M ed. (1992). "Algae". The Concise Dictionary of Botany. Oxford: Oxford University Press.
- ^ Skulberg OM (1996) "Terrestrial and limnic algae and cyanobacteria". In: A Catalogue of Svalvard Plants, Fungi, Algae and Cyanobacteria, Part 9, A Elvebakk and P Prestud (eds.) Norsk Polarinstitutt Skrifter, 198: 383-395.
- ^ Castenholz RA (1973) "Ecology of blue-green algae in hotsprings". In: The Biology of Blue-green algae. NG Carr and BA Whitton (eds), pp. 379-414. Blackwell Scientific Publications, Oxford.
- ^ Vasconcelos VM, Pereira E (2001). "Cyanobacteria diversity and toxicity in a Wastewater Treatment Plant (Portugal)". Water Research 35 (5): 1354–1357. doi:10.1016/S0043-1354(00)00512-1. PMID 11268858.
- ^ Gerald Karp (19 October 2009). Cell and Molecular Biology: Concepts and Experiments. John Wiley and Sons. pp. 14–. ISBN 9780470483374. http://books.google.com/books?id=arRGYE0GxRQC&pg=PA14. Retrieved 26 January 2011.
- ^ Herrero A and Flores E (editor). (2008). The Cyanobacteria: Molecular Biology, Genomics and Evolution. Caister Academic Press. ISBN 978-1-904455-15-8.
- ^ a b c d e Sinonen K and Jones G (1999) "Cyanobacterial Toxins" In Toxic Cyanobacteria in Water. Chorus I and Bartram J (eds): 41-111. WHO, Geneva.
- ^ Lindsey R and Scott M (2010) What are phytoplankton NASA Earth Observatory.
- ^ a b c d Harmful algal blooms in the Great Lakes 2009, NOAA, Center of Excellence for Great Lakes and Human Health.
- ^ a b Stewart I and Falconer IR (2008) "Cyanobacteria and cyanobacterial toxins" Pages 271–296 in Oceans and human health: risks and remedies from the seas, Eds: Walsh PJ, Smith SL and Fleming LE. Academic Press, ISBN 0123725844.
- ^ Moestrup Ø, Akselman R, Cronberg G, Elbraechter M, Fraga S, Halim Y, Hansen G, Hoppenrath M, Larsen J, Lundholm N, Nguyen LN and Zingone A. "IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae (HABs)" Accessed 21 January 2011.
- ^ a b Vasconcelos V (2006). "Eutrophication, toxic cyanobacteria and cyanotoxins: when ecosystems cry for help" (PDF). Limnetica 25 (1–2): 425–432. http://www.limnetica.net/Limnetica/limne25a/L25a425_Eutrophication_toxic_cyanobacteria_cyanotoxins.pdf.
- ^ Forc, N.S.W.B.G.A.T. (1992). "Final report of the NSW Blue-Green Algae Task Force". Parramatta: NSW Department of Water Resources.
- ^ Herath, G. (1995). "The algal bloom problem in Australian waterways: an economic appraisal". Review of Marketing and Agricultural Economics 63 (1): 77–86.
- ^ a b c d e f g Chorus & Bartram, 1999
- ^ Pelaez M et al, 2010
- ^ Sivonen K, Kononen K, Carmichael WW, Dahlem AM, Rinehart KL, Kiviranta J, Niemela SI (1989). "Occurrence of the hepatotoxic cyanobacterium Nodularia spumigena in the Baltic Sea and structure of the toxin". Appl. Environ. Microbiol. 55 (8): 1990–5. PMC 202992. PMID 2506812. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=202992.
- ^ David P. Fewer DP, Köykkä K, Halinen K, Jokela J, Lyra C, Sivonen K (2009). "Culture-independent evidence for the persistent presence and genetic diversity of microcystin-producing Anabaena (Cyanobacteria) in the Gulf of Finland". Environmental Microbiology 11 (4): 855–866. doi:10.1111/j.1462-2920.2008.01806.x. PMID 19128321.
- ^ Rhoades, David F (1979). "Evolution of Plant Chemical Defense against Herbivores". In Rosenthal, Gerald A., and Janzen, Daniel H. Herbivores: Their Interaction with Secondary Plant Metabolites. New York: Academic Press. p. 41. ISBN 0-12-597180-X.
- ^ Carmichael WW, Gorham PR (1978). "Anatoxins from clones of Anabaena flos-aquae isolated from lakes of western Canada." Mitt. Infernal. Verein. Limnol". , 21: 285–295.
- ^ Carmichael WW, Biggs DF, Gorham PR (1975). "Toxicology and pharmacological action of Anabaena flos-aquae toxin". Science 187 (4176): 542–544. doi:10.1126/science.803708. PMID 803708.
- ^ Wood S. A., Rasmussen J. P., Holland P. T., Campbell R., Crowe A. L. M. (2007). "First Report of the Cyanotoxin Anatoxin-A from Aphanizomenon issatschenkoi (cyanobacteria)". Journal of Phycology 43 (2): 356–365. doi:10.1111/j.1529-8817.2007.00318.x.
- ^ National Center for Environmental Assessment. "Toxicological Reviews of Cyanobacterial Toxins: Anatoxin-a" NCEA-C-1743
- ^ Devlin JP, Edwards OE, Gorham PR, Hunter NR, Pike RK, Stavric B (1977). "Anatoxin-a, a toxic alkaloid from Anabaena flos-aquae NRC-44h". Can. J. Chem. 55 (8): 1367–1371. doi:10.1139/v77-189. http://article.pubs.nrc-cnrc.gc.ca/ppv/RPViewDoc?issn=1480-3291&volume=55&issue=8&startPage=1367.
- ^ Moore RE (1977). "Toxins from blue-green algae". BioScience 27 (12): 797–802. doi:10.2307/1297756. JSTOR 1297756.
- ^ Metcalf JS and Codd GA (2009) "Cyanobacteria, neurotoxins and water resources: Are there implications for human neurodegenerative disease?" Informa Healthcare, 10(s2): 74-78.
- ^ Byth S (July 1980). "Palm Island mystery disease". The Medical Journal of Australia 2 (1): 40, 42. PMID 7432268.
- ^ Griffiths DJ, Saker ML (2003). "The Palm Island mystery disease 20 years on: a review of research on the cyanotoxin cylindrospermopsin". Environ Toxicol 18 (2): 78–93. doi:10.1002/tox.10103. PMID 12635096.
- ^ Kinnear S (2010) Cylindrospermopsin: A Decade of Progress on Bioaccumulation Research Marine Drugs 8: 542-564; doi:10.3390/md8030542
- ^ Clark RF, Williams SR, Nordt SP, Manoguerra AS (1999). "A review of selected seafood poisonings". Undersea Hyperb Med 26 (3): 175–84. PMID 10485519. http://archive.rubicon-foundation.org/2314. Retrieved 2008-08-12.
- ^ Nakamuraa M, Oshimaa Y, Yasumoto T (1984). "Occurrence of saxitoxin in puffer fish". Toxicon 22 (3): 381–385. doi:10.1016/0041-0101(84)90082-5. PMID 6474491.
- ^ Landsberg JH, 2002. The effects of harmful algal blooms on aquatic organisms. Reviews in Fisheries Science, 10(2): 113–390.
- ^ Kao CY and Levinson SR (1986) Tetrodotoxin, saxitoxin, and the molecular biology of the sodium channel New York Academy of Sciences. ISBN 0897663543.
- ^ Chemical Weapons Convention: Schedule 1 Organisation for the Prohibition of Chemical Weapons, The Hague, Netherlands. Retrieved 26 January 2011.
- ^ Wallace R, Melton HK and Schlesinger HR (2009) Spycraft: the secret history of the CIA's spytechs from communism to Al-Qaeda. Penguin Group USA, ISBN 0452295475.
- ^ Stewart I, Schluter PJ, Shaw GR (2006). "Cyanobacterial lipopolysaccharides and human health - a review". Environ Health 5 (1): 7. doi:10.1186/1476-069X-5-7. PMC 1489932. PMID 16563160. http://www.ehjournal.net/content/5/1/7.
References
- Chorus I and Bartram J (1999) Toxic cyanobacteria in water: A guide to their public health consequences, monitoring and management World Health Organisation. E & FN Spon, ISBN 0-419-23930-8.
- Pelaez M et al. (2010) "Sources and Occurrence of Cyanotoxins Worldwide". In Xenobiotics in the Urban Water Cycle, Environmental Pollution, 16(I): 101-127, DOI: 10.1007/978-90-481-3509-7_6
External links
- Cyanosite - A Webserver for Cyanobacterial Research, Purdue University.
- Dangers of toxic algae Environment Canterbury Updated 31 October 2009. Retrieved 23 January 2011.
Categories:- Cyanotoxins
Wikimedia Foundation. 2010.