- Chemokine receptor
-
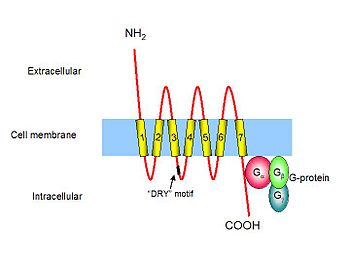
Chemokine receptors are cytokine receptors found on the surface of certain cells, which interact with a type of cytokine called a chemokine.[1][2] There have been 19 distinct chemokine receptors described in mammals. They each have a 7 transmembrane (7TM) structure and couple to G-protein for signal transduction within a cell, making them members of a large protein family of G protein-coupled receptors. Following interaction with their specific chemokine ligands, chemokine receptors trigger a flux in intracellular calcium (Ca2+) ions (calcium signaling). This causes cell responses, including the onset of a process known as chemotaxis that traffics the cell to a desired location within the organism. Chemokine receptors are divided into different families, CXC chemokine receptors, CC chemokine receptors, CX3C chemokine receptors and XC chemokine receptors that correspond to the 4 distinct subfamilies of chemokines they bind.
Contents
Structural characteristics
Chemokine receptors are G protein-coupled receptors containing 7 transmembrane domains that are found predominantly on the surface of leukocytes. Approximately 19 different chemokine receptors have been characterized to date, which share many common structural features; they are composed of about 350 amino acids that are divided into a short and acidic N-terminal end, seven helical transmembrane domains with three intracellular and three extracellular hydrophilic loops, and an intracellular C-terminus containing serine and threonine residues that act as phosphorylation sites during receptor regulation. The first two extracellular loops of chemokine receptors are linked together by disulfide bonding between two conserved cysteine residues. The N-terminal end of a chemokine receptor binds to chemokine(s) and is important for ligand specificity. G-proteins couple to the C-terminal end, which is important for receptor signaling following ligand binding. Although chemokine receptors share high amino acid identity in their primary sequences, they typically bind a limited number of ligands.[3]
Signal transduction
For more details on this topic, see G protein.Intracellular signaling by chemokine receptors is dependent on neighbouring G-proteins. G-proteins exist as a heterotrimer; they are composed of three distinct subunits. When the molecule GDP is bound to the G-protein subunit, the G-protein is in an inactive state. Following binding of the chemokine ligand, chemokine receptors associate with G-proteins, allowing the exchange of GDP for another molecule called GTP, and the dissociation of the different G protein subunits. The subunit called Gβ activates an enzyme known as Phospholipase C (PLC) that is associated with the cell membrane. PLC cleaves Phosphatidylinositol (4,5)-bisphosphate (PIP2) to form two second messenger molecules called inositol triphosphate (IP3) and diacylglycerol (DAG); DAG activates another enzyme called protein kinase C (PKC), and IP3 triggers the release of calcium from intracellular stores. These events promote many signaling cascades, effecting a cellular response. For example, when CXCL8 (IL-8) binds to its specific receptors, CXCR1 or CXCR2, a rise in intracellular calcium activates the enzyme phospholipase D (PLD) that goes on to initiate an intracellular signaling cascade called the MAP kinase pathway. At the same time the G-protein subunit Gα directly activates an enzyme called protein tyrosine kinase (PTK), which phosphorylates serine and threonine residues in the tail of the chemokine receptor causing its desensitisation or inactivation. The initiated MAP kinase pathway activates specific cellular mechanisms involved in chemotaxis, degranulation, release of superoxide anions and changes in the avidity of cell adhesion molecules called integrins.[3]
Families
- CXC chemokine receptors (seven members)
- CC chemokine receptors (ten/eleven members)
- C chemokine receptors (one member, XCR1)
- CX3C chemokine receptors (one member, CX3CR1)
References
- ^ Murphy PM, Baggiolini M, Charo IF, Hébert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA (2000). "International union of pharmacology. XXII. Nomenclature for chemokine receptors" (abstract page). Pharmacol. Rev. 52 (1): 145–76. PMID 10699158. http://pharmrev.aspetjournals.org/cgi/content/abstract/52/1/145.
- ^ Murphy PM (2002). "International Union of Pharmacology. XXX. Update on chemokine receptor nomenclature". Pharmacol. Rev. 54 (2): 227–9. doi:10.1124/pr.54.2.227. PMID 12037138.
- ^ a b Murdoch C, Finn A (2000). "Chemokine receptors and their role in inflammation and infectious diseases". Blood 95 (10): 3032–43. PMID 10807766.
External links
- "Chemokine Receptors". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology. http://www.iuphar-db.org/GPCR/ChapterMenuForward?chapterID=1280.
- The Cytokine Receptor Database
Cytokine receptors Chemokine receptor
(GPCRs)OtherTNF receptor 1-1011-2021-25JAK-STAT OtherIg superfamily IL-17 family S/T B trdu: iter (nrpl/grfl/cytl/horl), csrc (lgic, enzr, gprc, igsr, intg, nrpr/grfr/cytr), itra (adap, gbpr, mapk), calc, lipd; path (hedp, wntp, tgfp+mapp, notp, jakp, fsap, hipp, tlrp) Categories:- Chemokine receptors
- Cell biology
- Integral membrane proteins
Wikimedia Foundation. 2010.