- Sarcosine
-
Sarcosine 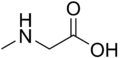 2-(Methylamino)acetic acidOther namesSarcosine
2-(Methylamino)acetic acidOther namesSarcosine
N-MethylglycineIdentifiers CAS number 107-97-1 
ChemSpider 1057 
UNII Z711V88R5F 
EC-number 203-538-6 KEGG C00213 
ChEBI CHEBI:15611 
ChEMBL CHEMBL304383 
Jmol-3D images Image 1 - O=C(O)CNC
Properties Molecular formula C3H7NO2 Molar mass 89.093 g/mol Melting point 208-212 °C decomp.
Acidity (pKa) 2.23 (carboxyl), 10.01 (amino)[1]  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Sarcosine, also known as N-methylglycine, is an intermediate and byproduct in glycine synthesis and degradation. Sarcosine is metabolized to glycine by the enzyme sarcosine dehydrogenase, while glycine-N-methyl transferase generates sarcosine from glycine. Sarcosine is a natural amino acid found in muscles and other body tissues. In the laboratory, it may be synthesized from chloroacetic acid and methylamine. Sarcosine is found naturally as an intermediate in the metabolism of choline to glycine. Sarcosine is sweet to the taste and dissolves in water. It is used in manufacturing biodegradable surfactants and toothpastes as well as in other applications.
Sarcosine is ubiquitous in biological materials and is present in such foods as egg yolks, turkey, ham, vegetables, legumes, etc.
Sarcosine is formed from dietary intake of choline and from the metabolism of methionine, and is rapidly degraded to glycine, which, in addition to its importance as a constituent of protein, plays a significant role in various physiological processes as a prime metabolic source of components of living cells such as glutathione, creatine, purines and serine. The concentration of sarcosine in blood serum of normal human subjects is 1.59 ± 1.08 nanomolar.[2]
Contents
Clinical significance
Sarcosine has no known toxicity, as evidenced by the lack of phenotypic manifestations of sarcosinemia, an inborn error of sarcosine metabolism. Sarcosinemia can result from severe folate deficiency because of the folate requirement for the conversion of sarcosine to glycine.
Schizophrenia
Recently, sarcosine has been investigated in relation to the mental illness schizophrenia. Early evidence suggests that intake of 2 g/day sarcosine as add-on therapy to certain antipsychotics (not clozapine[3]) in schizophrenia gives significant additional reductions in both positive and negative symptomatology as well as the neurocognitive and general psychopathological symptoms that are common to the illness. Sarcosine had been tolerated well.[4] It is also under investigation for the possible prevention of schizophrenic illness during the prodromal stage of the disease. It acts as a type 1 glycine transporter inhibitor and a glycine agonist. It increases glycine concentrations in the brain thus causing increased NMDA receptor activation and a reduction in symptoms. As such, it might be an interesting treatment option and a possible new direction in the treatment of the mental illness in the future.
Depression
Major depressive disorder is a complex disease and most currently available antidepressants aiming at monoamine neurotransmission exhibit limited efficacy and cognitive effects. N-methyl-D-aspartate (NMDA), one subtype of glutamate receptors, plays an important role in learning and memory. N-methyl-D-aspartic acid (NMDA) enhancing agents, such as Sarcosine (N-methylglycine), have been used as adjunctive therapy of schizophrenia. Preliminary clinic trials indicated that intake of Sarcosine improved not only psychotic but also depressive symptoms in patients with schizophrenia.[5]
Prostate cancer marker
In a paper published in the journal Nature in 2009, sarcosine was reported to activate prostate cancer cells and to indicate the malignancy of prostate cancer cells when measured in urine.[6] Sarcosine was identified as a differential metabolite that was greatly increased during prostate cancer progression to metastasis and could be detected in urine. Sarcosine levels were also increased in invasive prostate cancer cell lines relative to benign prostate epithelial cells.[7] Sarcosine levels seemed to control the invasiveness of the cancer.[6]
However, this conclusion has been disputed. A German research team reported a different result in 2010.[8] After measuring sarcosine levels in urine samples from prostate cancer patients, they concluded that measuring sarcosine in urine fails as a marker in prostate cancer detection and identification of aggressive tumors. In addition, another report concluded that serum sarcosine is not a marker for prostate cancer.[9] A review of the literature reached a similar conclusion.[10]
History
Sarcosine was first isolated and named by the German chemist Justus von Liebig in 1847, while Jacob Volhard first synthesized it in 1862.
Volhard successfully synthesized the compound while working the lab of Hermann Kolbe. Prior to the synthesis of sarcosine, it was long believed to be hydrosolate of creatine, a compound found in meat extract. Under this assumption, Volhard proposed that sarcosine was N-methylglycine, and proved so by preparing the compound with methylamine and monochloroacetic acid.[11]
See also
References
- ^ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ^ Allen, RH; Stabler, SP; Lindenbaum, J (1993). "Serum betaine, N,N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related inborn errors of metabolism". Metabolism: clinical and experimental 42 (11): 1448–60. doi:10.1016/0026-0495(93)90198-W. PMID 7694037.
- ^ Lane H, Huang C, Wu P, Liu Y, Chang Y, Lin P, Chen P, Tsai G (2006). "Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to clozapine for the treatment of schizophrenia". Biol Psychiatry 60 (6): 645–9. doi:10.1016/j.biopsych.2006.04.005. PMID 16780811.
- ^ Tsai G, Lane H, Yang P, Chong M, Lange N (2004). "Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia". Biol Psychiatry 55 (5): 452–6. doi:10.1016/j.biopsych.2003.09.012. PMID 15023571.
- ^ http://clinicaltrials.gov/ct2/show/NCT00977353 Clinicaltrials.gov "N-methylglycine (Sarcosine) Treatment for Depression"
- ^ a b Sreekumar, Arun; Poisson, Laila M.; Rajendiran, Thekkelnaycke M.; Khan, Amjad P.; Cao, Qi; Yu, Jindan; Laxman, Bharathi; Mehra, Rohit et al. (2009). "Metabolomic Profiles Delineate Potential Role for Sarcosine in Prostate Cancer Progression". Nature 457 (7231): 910–4. doi:10.1038/nature07762. PMC 2724746. PMID 19212411. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2724746.
- ^ A Urine Test for Prostate Cancer?, Jennifer Couzin, Science NOW, 11 February 2009
- ^ F. Jentzmik et al. (2010). "Sarcosine in Urine after Digital Rectal Examination Fails as a Marker in Prostate Cancer Detection and Identification of Aggressive Tumours". European Urology 58 (1): 12–18. doi:10.1016/j.eururo.2010.01.035. PMID 20117878. http://www.europeanurology.com/article/S03022838(10)000849/pdf/Sarcosine+in+Urine+after+Digital+Rectal+Examination+Fails+as+a+Marker+in+Prostate+Cancer+Detection+and+Identification+of+Aggressive+Tumours.
- ^ E. A Struys et al. (2010). "Serum sarcosine is not a marker for prostate cancer". Annals Clinical Biochemistry 47 (Pt 3): 282. doi:10.1258/acb.2010.009270. PMID 20233752. http://acb.rsmjournals.com/cgi/content/full/47/3/282#ACB-09-270C1.
- ^ M. Pavlou and E. P. Diamandis (2009). "The Search for New Prostate Cancer Biomarkers Continues". Clinical Chemistry 55 (7): 1277–1279. doi:10.1373/clinchem.2009.126870. PMID 19478024. http://www.clinchem.org/cgi/content/full/55/7/1277#R1.
- ^ http://publishing.cdlib.org/ucpressebooks/view?docId=ft5g500723&chunk.id=d0e7179&toc.depth=1&toc.id=d0e7179&brand=eschol University of California Press "The Quiet Revolution"
Neurotransmitters Amino acids Alanine · Aspartate · Cycloserine · DMG · GABA · Glutamate · Glycine · Hypotaurine · Kynurenic acid (Transtorine) · NAAG (Spaglumic acid) · NMG (Sarcosine) · Serine · Taurine · TMG (Betaine)
Endocannabinoids 2-AG · 2-AGE (Noladin ether) · AEA (Anandamide) · NADA · OAE (Virodhamine) · Oleamide · PEA (Palmitoylethanolamide) · RVD-Hpα · Hp (Hemopressin)
Gasotransmitters Monoamines Purines Trace amines 3-ITA · 5-MeO-DMT · Bufotenin · DMT · NMT · Octopamine · Phenethylamine · Synephrine · Thyronamine · Tryptamine · Tyramine
Others 1,4-BD · Acetylcholine · GBL · GHB · Histamine
See also Template:NeuropeptidesGlycinergics Receptor
ligandsAgonistsAlanine • Cycloserine • Dimethylglycine • Glycine • Hypotaurine • Methylglycine (Sarcosine) • Milacemide • Serine • Taurine • Trimethylglycine (Betaine)Reuptake
inhibitorsPlasmalemmalGlyT1 inhibitorsGlyT2 inhibitorsVIAAT InhibitorsEnzyme
inhibitorsSHMT InhibitorsGDC InhibitorsDAAO InhibitorsOthers Categories:- Amino acids
Wikimedia Foundation. 2010.
