- Hydrochlorothiazide
-
Hydrochlorothiazide 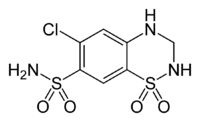
Systematic (IUPAC) name 6-chloro-1,1-dioxo-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide Clinical data Trade names Apo-hydro, Aquazide h, Dichlotride among others, Oretic AHFS/Drugs.com monograph MedlinePlus a682571 Pregnancy cat. B (D if used to treat pregnancy-induced hypertension) Legal status ℞ Prescription only Routes Oral (capsules, tablets, oral solution) Pharmacokinetic data Bioavailability Variably absorbed from GI tract Metabolism does not undergo significant metabolism (>95% excreted unchanged in urine)[1] Half-life 5.6-14.8 h Excretion Primarily excreted unchanged in urine Identifiers CAS number 58-93-5 
ATC code C03AA03 PubChem CID 3639 DrugBank DB00999 ChemSpider 3513 
UNII 0J48LPH2TH 
KEGG D00340 
ChEMBL CHEMBL435 
Chemical data Formula C7H8ClN3O4S2 Mol. mass 297.74 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Hydrochlorothiazide, abbreviated HCTZ, HCT, or HZT, is a first-line diuretic drug of the thiazide class that acts by inhibiting the kidneys' ability to retain water. This reduces the volume of the blood, decreasing blood return to the heart and thus cardiac output and, by other mechanisms, is believed to lower peripheral vascular resistance.[2] Hydrochlorothiazide is a calcium-sparing diuretic, meaning it can help the body get rid of excess water while still keeping calcium.
Contents
Medical uses
Hydrochlorothiazide is frequently used for the treatment of hypertension, congestive heart failure, symptomatic edema, diabetes insipidus, renal tubular acidosis, and the prevention of kidney stones.[3]
It is also sometimes used for hypercalciuria, Dent's disease and Ménière's disease. For diabetes insipidus, the effect of thiazide diuretics is presumably mediated by a hypovolemia-induced increase in proximal sodium and water reabsorption, thereby diminishing water delivery to the ADH-sensitive sites in the collecting tubules and reducing the urine output.
Thiazides are also used in the treatment of osteoporosis. Thiazides decrease mineral bone loss by promoting calcium retention in the kidney, and by directly stimulating osteoblast differentiation and bone mineral formation.[4]
It is frequently given together with losartan (an angiotensin II receptor antagonist) as hydrochlorothiazide/losartan.
Adverse effects
- Hypokalemia, an occasional side effect, can be usually prevented by potassium supplements or by combining hydrochlorothiazide with a potassium-sparing diuretic.
- Hypomagnesemia
- Hyperuricemia and gout
- High blood sugar
- Hyperlipidemia
- Hypercalcemia
- Headache
- Nausea/vomiting
- Photosensitivity
- Weight Gain
- Gout
May cause allergies in those with sulfa drugs allergies.
Mechanism of action
Hydrochlorothiazide belongs to the thiazide class of diuretics. It reduces blood volume by acting on the kidneys to reduce sodium (Na) reabsorption in the distal convoluted tubule. The major site of action in the nephron appears on an electroneutral Na+-Cl- co-transporter by competing for the chloride site on the transporter. By impairing Na transport in the distal convoluted tubule, hydrochlorothiazide induces a natriuresis and concomitant water loss. Thiazides increase the reabsorption of calcium in this segment in a manner unrelated to sodium transport.[5] Additionally, by other mechanisms, HCTZ is believed to lower peripheral vascular resistance.[6]
Brand names
Hydrochlorothiazide is sold both as a generic drug and under a large number of brand names, including Apo-Hydro, Aquazide H,Dichlotride, Hydrodiuril, HydroSaluric, Hydrochlorot, Microzide, Esidrex, and Oretic.
Hydrochlorothiazide is also used in combination with many popular drugs used to treat hypertension such as Diovan HCT, Zestoretic, Benicar HCT, Olmy-H, Atacand HCT, and Lotensin HCT, Temax-H and others.
Use with performance-enhancing drugs
Hydrochlorothiazide was detected in the urine of the Russian cyclist Alexandr Kolobnev during the 2011 Tour de France.[7]Kolobnev was the first cyclist to leave the 2011 race in connection with performance-enhancing drugs.[8]Although hydrochlorothiazide is not itself a performance-enhancing drug, it may be used to mask the use of performance-enhancing drugs, and is classed by the World Anti-Doping Agency as a "specified substance" (i.e. it is prohibited).[9][10]
See also
- Benicar HCT
- Diovan HCT
References
- ^ Beermann B, Groschinsky-Grind M, Rosén A. (1976). "Absorption, metabolism, and excretion of hydrochlorothiazide". Clin Pharmacol Ther 19 (5 (Pt 1)): 531–7.
- ^ Duarte JD, Cooper-DeHoff RM (June 2010). "Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics". Expert Rev Cardiovasc Ther 8 (6): 793–802. doi:10.1586/erc.10.27. PMC 2904515. PMID 20528637. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2904515.
- ^ "Hydrochlorothiazide". The American Society of Health-System Pharmacists. http://www.drugs.com/monograph/hydrochlorothiazide.html. Retrieved 3 April 2011.
- ^ Dvorak MM, De Joussineau C, Carter DH, et al. (2007). "Thiazide diuretics directly induce osteoblast differentiation and mineralized nodule formation by targeting a NaCl cotransporter in bone". J. Am. Soc. Nephrol. 18 (9): 2509–16. doi:10.1681/ASN.2007030348. PMC 2216427. PMID 17656470. http://jasn.asnjournals.org/cgi/pmidlookup?view=long&pmid=17656470.
- ^ Uniformed Services University Pharmacology Note Set #3 2010, Lectures #39 & #40, Eric Marks
- ^ Duarte, Cooper-DeHoff Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics Expert Rev Cardiovasc Ther. Author manuscript; available in PMC 2011 April 1. Published in final edited form as: Expert Rev Cardiovasc Ther. 2010 June; 8(6): 793–802. doi: 10.1586/erc.10.27 PMCID: PMC2904515 NIHMSID: NIHMS215063 Free full text: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2904515/?tool=pubmed
- ^ "Tour de France: Alexandr Kolobnev positive for banned diuretic". Velonation. 2011-07-11. Archived from the original on 2011-07-12. http://www.webcitation.org/607VO9Grh. Retrieved 2011-07-12.
- ^ "Kolobnev denies knowledge of doping product, says not fired by Katusha". Velonation. 2011-07-12. Archived from the original on 2011-07-12. http://www.webcitation.org/607VRWuBj. Retrieved 2011-07-12.
- ^ "Press release: Adverse Analytical Finding for Kolobnev". Union Cycliste Internationale. 2011-07-11. Archived from the original on 2011-07-12. http://www.webcitation.org/607TqzrHW. Retrieved 2011-07-12.
- ^ "Kolobnev Tour de France's first doping case". Cycling News (Bath, UK: Future Publishing Limited). 2011-07-11. Archived from the original on 2011-07-12. http://www.webcitation.org/607TfSxj5. Retrieved 2011-07-12.
External links
- NIH medlineplus druginfo
- U.S. National Library of Medicine: Drug Information Portal - Hydrochlorothiazide
Symporter inhibitors Sodium chloride thiazide: Bendroflumethiazide • Chlorothiazide • Cyclopenthiazide • Cyclothiazide • Hydrochlorothiazide • Hydroflumethiazide • Methyclothiazide • Polythiazide • Trichlormethiazide
other: Chlorthalidone • MetolazoneSodium, potassium, chloride Antihypertensives: diuretics (C03) Sulfonamides
(except EA)Hydrochlorothiazide# • Bendroflumethiazide • Hydroflumethiazide • Chlorothiazide • Polythiazide • Trichlormethiazide • Cyclopenthiazide • Methyclothiazide • Cyclothiazide • MebutizideQuinethazone • Clopamide • Chlortalidone • Mefruside • Clofenamide • Metolazone • Meticrane • Xipamide • Indapamide • Clorexolone • FenquizonePotassium-sparing (at CD) ESC blockersOsmotic diuretics (PT, DL) VAs (DCT and CD) Other Categories:- Thiazides
- World Health Organization essential medicines
- Benzothiadiazines
Wikimedia Foundation. 2010.

