- Losartan
-
Losartan 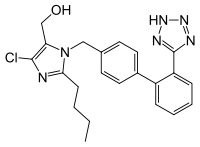
Systematic (IUPAC) name (2-butyl-4-chloro-1-{[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1H-imidazol-5-yl)methanol Clinical data Trade names Cozaar AHFS/Drugs.com monograph MedlinePlus a695008 Licence data US FDA:link Pregnancy cat. D(AU) D(US) Legal status ℞ Prescription only Routes Oral Pharmacokinetic data Bioavailability 25–35% Metabolism Hepatic (CYP2C9, CYP3A4) Half-life 1.5–2 hours Excretion Renal 13–25%, biliary 50–60% Identifiers CAS number 114798-26-4 
ATC code C09CA01 PubChem CID 3961 IUPHAR ligand 590 DrugBank APRD00052 ChemSpider 3824 
UNII JMS50MPO89 
KEGG D08146 
ChEBI CHEBI:6541 
ChEMBL CHEMBL191 
Chemical data Formula C22H23ClN6O Mol. mass 422.91 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Losartan (rINN) (
 /loʊˈsɑrtən/) is an angiotensin II receptor antagonist drug used mainly to treat high blood pressure (hypertension). Losartan was the first angiotensin II receptor antagonist to be marketed. Losartan potassium is marketed by Merck & Co. Inc. under the trade name Cozaar. As of 2009[update], losartan is available in generic form.
/loʊˈsɑrtən/) is an angiotensin II receptor antagonist drug used mainly to treat high blood pressure (hypertension). Losartan was the first angiotensin II receptor antagonist to be marketed. Losartan potassium is marketed by Merck & Co. Inc. under the trade name Cozaar. As of 2009[update], losartan is available in generic form.As with all angiotensin II type 1 receptor (AT1) antagonists, losartan is indicated for the treatment of hypertension. It may also delay progression of diabetic nephropathy, and is also indicated for the reduction of renal disease progression in patients with type 2 diabetes, hypertension and microalbuminuria (>30 mg/24 hours) or proteinuria (>900 mg/24 hours).[citation needed]
Although clinical evidence shows calcium channel blockers and thiazide-type diuretics are preferred first-line treatments for most patients (from both efficacy and cost points of view), an angiotensin II receptor antagonist such as losartan is recommended as first-line treatment in patients under the age of 55 who cannot tolerate an ACE inhibitor.[1] The LIFE study demonstrated losartan was significantly superior to atenolol in the primary prevention of adverse cardiovascular events (myocardial infarction or stroke), with a significant reduction in cardiovascular morbidity and mortality for a comparable reduction in blood pressure. A study hints that losartan has a beneficial effect on mitochondria by reversing age related dysfunction in maintaining normal blood pressure and cellular energy usage.[2][3]
Contents
Combination with diuretic
Further information: Hydrochlorothiazide/losartanLosartan is available as hydrochlorothiazide/losartan, a combination drug with a low dose thiazide diuretic to achieve an additive antihypertensive effect.
Pharmacokinetics
Losartan is well absorbed following oral administration and undergoes significant first-pass metabolism to produce 5-carboxylic acid metabolite, designated as EXP3174. This metabolite is a long-acting (6 to 8 hr), noncompetitive antagonist at the AT1 receptor, and contributes to the pharmacological effects of losartan. EXP3174 is 10-40 times more potent in blocking AT1 receptors than losartan. Losartan's bioavailability is about 32%.
Metabolism is primarily by cytochrome P450 isoenzymes CYP2C9 and CYP3A4. Peak plasma concentrations of losartan and E-3174 occur about one hour and three to four hours, respectively, after an oral dose. Both losartan and E-3174 are more than 98% bound to plasma proteins. Losartan is excreted in the urine, and in the feces via bile, as unchanged drug and metabolites. About 4% of an oral dose is excreted unchanged in urine, and about 6% is excreted in urine as the active metabolite. The terminal elimination half lives of losartan and E-3174 are about 1.5 to 2.5 hours and 3 to 9 hours, respectively.
Research
Losartan has been found to downregulate the expression of transforming growth factor beta (TGF-β) types I and II receptors in the kidney of diabetic rats, which may partially account for its nephroprotective effects.[4] Effects on TGF-β expression may also account for its potential efficacy in Marfan syndrome and Duchenne muscular dystrophy (DMD) – losartan has been shown to prevent aortic aneurysm and certain pulmonary complications in a mouse model of the disease.[5]
Losartan is being studied[when?] for use in the treatment of the 20% of breast cancer tumors positive for AGTR1. The University of Michigan Comprehensive Cancer Center announced[when?] the result of an animal study which found losartan to "block" - reverse neoplastic changes - caused by this gene.[6]
Mechanism of action and pharmacological actions
Losartan is a selective, competitive angiotensin II receptor type 1 (AT1) receptor antagonist, reducing the end organ responses to angiotensin II. Losartan administration results in a decrease in total peripheral resistance (afterload) and cardiac venous return (preload) All of the physiological effects of angiotensin II, including stimulation of release of aldosterone, are antagonized in the presence of losartan. Reduction in blood pressure occurs independently of the status of the renin-angiotensin system. As a result of losartan dosing, plasma renin activity increases due to removal of the angiotensin II feedback.
Other uses
Losartan is a uricosuric. Because losartan can cause hyperkalemia, potassium supplements or salt substitutes containing potassium should not be used without consulting the prescribing physician.
Losartan is being[when?] researched[7][8] as a possible drug for marked slowing of aortic enlargement in Marfan and related syndromes.
Losartan is being researched as a possible protection against loss of damaged or old muscle.[9]
Losartan has recently been found to be a cognitive enhancer. It improved memory in people with normal blood pressure under standard conditions, as well as during memory impaired tasks (co-administration of scopolamine). [10]
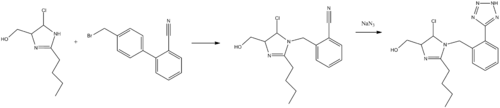
Synthesis
Carini, D. J.; Duncia, J. V.; Wong, P. C. B; 1992, U.S. Patent 5,138,069.
See also
References
- ^ http://www.nice.org.uk/nicemedia/pdf/CG034NICEguideline.pdf, p19
- ^ "Switch in cell's 'power plant' declines with age; rejuvenated by drug". Johns Hopkins Medicine. August 16, 2011. http://www.hopkinsmedicine.org/news/media/releases/switch_in_cells_power_plant_declines_with_age_rejuvenated_by_drug.
- ^ Abadir, P. M.; Foster, D. B.; Crow, M.; Cooke, C. A.; Rucker, J. J.; Jain, A.; Smith, B. J.; Burks, T. N. et al. (2011). "Identification and characterization of a functional mitochondrial angiotensin system". Proceedings of the National Academy of Sciences. doi:10.1073/pnas.1101507108.
- ^ Guo ZX, Qiu MC (June 2003). "[Losartan downregulates the expression of transforming growth factor beta type I and type II receptors in kidney of diabetic rat] [Losartan downregulates the expression of transforming growth factor beta type I and type II receptors in kidney of diabetic rat]" (in Chinese). Zhonghua Nei Ke Za Zhi 42 (6): 403–8. PMID 12895325.
- ^ Habashi, JP; Judge, DP; Holm, TM; Cohn, RD; Loeys, BL; Cooper, TK; Myers, L; Klein, EC et al. (April 2006). "Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome". Science 312 (5770): 117–21. Bibcode 2006Sci...312..117H. doi:10.1126/science.1124287. PMC 1482474. PMID 16601194. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1482474.
- ^ Rhodes, DR; Ateeq, B; Cao, Q; Tomlins, SA; Mehra, R; Laxman, B; Kalyana-Sundaram, S; Lonigro, RJ et al. (June 2009). "AGTR1 overexpression defines a subset of breast cancer and confers sensitivity to losartan, an AGTR1 antagonist". Proceedings of the National Academy of Sciences 106 (25): 10284–9. Bibcode 2009PNAS..10610284R. doi:10.1073/pnas.0900351106. PMC 2689309. PMID 19487683. http://www.pnas.org/content/106/25/10284.full.pdf.
Related news articles: "Breast Cancer Gene Can Be Blocked By Blood Pressure Drug" (Press release). ScienceDaily. June 7, 2009. http://www.sciencedaily.com/releases/2009/06/090601182651.htm. - ^ Molecular Determinants of Aortic Aneurysm and Other Manifestations of Connective Tissue Disorders
- ^ Weinberg, Marc S.; Adam J. Weinberg, Raymond B Cord, Horace Martin (2003). "P-609: Regression of dilated aortic roots using supramaximal and usual doses of angiotensin receptor blockers". American Journal of Hypertension 16. http://www.nature.com/ajh/journal/v16/n1s/abs/ajh2003891a.html. Retrieved 2011-11-02. "In conclusion, we demonstrated regression of DAR using ARBs at moderate and supramaximal doses. Intensive ARB therapy offers a promise to reduce the natural progression of disease in patients with DARs."
- ^ Burks, TN; Andres-Mateos, E; Marx, R; Mejias, R; Van Erp, C; Simmers, JL; Walston, JD; Ward, CW et al. (May 11, 2011). "Losartan Restores Skeletal Muscle Remodeling and Protects Against Disuse Atrophy in Sarcopenia". Science Translational Medicine 3 (82): 82ra37. doi:10.1126/scitranslmed.3002227.
Related news articles: "Blood Pressure Drug Shows Some Muscle" (Press release). Johns Hopkins. May 11, 2011. http://www.hopkinsmedicine.org/news/media/releases/blood_pressure_drug_shows_some_muscle. - ^ Mechaeil, R (September 2011). "Cognitive enhancement following acute losartan in normotensive young adults.". Psychopharmacology 217 (1): 51–60. doi:10.1007/s00213-011-2257-9. PMID 21484242.
External links
Merck & Co., Inc. Corporate directors: Richard Clark · Johnnetta Cole · William Harrison · William Kelley · Rochelle Lazarus · Thomas Shenk · Anne Tatlock · Samuel Thier · Wendell Weeks · Peter WendellProducts: Alendronate · Aprepitant · Ertapenem · Ezetimibe · Ezetimibe/simvastatin · Finasteride · Fosaprepitant · Indinavir · Losartan · Lovastatin · Montelukast · Raltegravir · Rizatriptan · Rofecoxib · Simvastatin · Sitagliptin · VorinostatPublications: The Merck Manuals (Index · Manual · Veterinary · USD ( 2% FY 2004) · Employees: 63,000 · Stock Symbol: NYSE: MRK · Website: merck.com
2% FY 2004) · Employees: 63,000 · Stock Symbol: NYSE: MRK · Website: merck.comAntihypertensives: agents acting on the renin-angiotensin system (C09) ACE inhibitors
("-pril")Sulfhydryl-containing: Captopril • Zofenopril
Dicarboxylate-containing: Enalapril# • Ramipril • Quinapril • Perindopril • Lisinopril (+HCT) • Benazepril
Phosphonate-containing: Fosinopril
Other/ungrouped: Alacepril • Cilazapril • Delapril • Imidapril • Moexipril • Rentiapril • Spirapril • Temocapril • TrandolaprilAIIRAs/
("-sartan")Azilsartan • Candesartan • Eprosartan • Irbesartan • Losartan • Olmesartan • Tasosartan§ • Telmisartan (+HCT) • Valsartan (+HCT)Renin inhibitors/
("-kiren")#WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III Categories:- Alcohols
- Angiotensin II receptor antagonists
- Imidazoles
- Organochlorides
- Tetrazoles
Wikimedia Foundation. 2010.