- Olmesartan
-
Olmesartan medoxomil 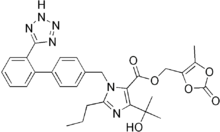
Systematic (IUPAC) name (5-methyl-2-oxo-2H-1,3-dioxol-4-yl)methyl 4-(2-hydroxypropan-2-yl)-2-propyl-1-({4-[2-(2H-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)-1H-imidazole-5-carboxylate Clinical data Trade names Benicar AHFS/Drugs.com monograph MedlinePlus a603006 Pregnancy cat. C (D if used in second or third trimester) Legal status ℞ Prescription only Routes Oral Pharmacokinetic data Bioavailability 26% Metabolism Hepatic (cannot be removed by hemodialysis) Half-life 13 hours Excretion Renal 40%, biliary 60% Identifiers CAS number 144689-63-4 
ATC code C09CA08
C09DA08 (with diuretics)
C09DB02 (with amlodipine)PubChem CID 130881 DrugBank APRD00223 ChemSpider 115748 
UNII 6M97XTV3HD 
KEGG D01204 
ChEMBL CHEMBL1516 
Chemical data Formula C29H30N6O6 Mol. mass 558.585 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Olmesartan medoxomil (trade names: Benicar in the US, Olmetec in EU and Canada, WinBP, Golme in India, Erastapex in Egypt) is an angiotensin II receptor antagonist used to treat high blood pressure.
Contents
Indications
Olmesartan is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents.[1] The U.S. Food and Drug Administration (FDA) has determined that the benefits of Benicar continue to outweigh its potential risks when used for the treatment of patients with high blood pressure according to the drug label.[2]
Contraindications
Contraindications for treatment with olmesartan include biliary obstruction (BNF).
Cautions
Angiotensin-II receptor antagonists should be used with caution in renal artery stenosis. Monitoring of plasma-potassium concentration is advised, particularly in the elderly and in patients with renal impairment; lower initial doses may be appropriate in these patients. Angiotensin-II receptor antagonists should be used with caution in aortic or mitral valve stenosis and in hypertrophic cardiomyopathy. Those with primary aldosteronism, and Afro-Caribbean patients (particularly those with left ventricular hypertrophy), may not benefit from an angiotensin-II receptor antagonist.
Adverse effects
The incidence of adverse effects with BENICAR (the trade name for olmesartan medoxomil) is reported as similar to placebo; the only adverse effect that occurred in >1% of patients treated with BENICAR and more frequently than placebo was dizziness (3% vs 1%). The full prescribing information for Benicar notes that as with all drugs that act directly on the renin-angiotensin system, olmesartan is contraindicated in pregnancy and can cause injury and even death to the developing fetus. In studies of angiotensin II receptor antagonists such as olmesartan, patients with unilateral or bilateral renal artery stenosis, increases in serum creatinine or blood urea nitrogen (BUN) have been reported. There has been no long-term use of olmesartan medoxomil in patients with unilateral or bilateral renal artery stenosis, but similar results may be expected.[3]
Structure
The olmesartan molecule includes one tetrazole group (a 5-member heterocyclic ring of four nitrogen and one carbon atom) and one imidazole group (a 5-membered planar heterocyclic aromatic ring of two nitrogen and three carbon atoms, classified as an alkaloid).
Mechanism of action
Olmesartan is a prodrug that works by blocking the binding of angiotensin II to the AT1 receptors in vascular muscle; it is therefore independent of angiotensin II synthesis pathways, unlike ACE inhibitors. By blocking the binding rather than the synthesis of angiotensin II, olmesartan inhibits the negative regulatory feedback on renin secretion. As a result of this blockage, olmesartan reduces vasoconstriction and the secretion of aldosterone. This lowers blood pressure by producing vasodilation, and decreasing peripheral resistance.
Interactions
Do not use non-prescription products that contain stimulants; including diet pills, and cold medicines. Consult your doctor before taking any potassium supplements, including salt substitutes.
Dosage and administration
The usual recommended starting dose of olmesartan is 20 mg once daily. The dose may be increased to 40 mg after 2 weeks of therapy, if further reduction in blood pressure is desirable. Doses above 40 mg do not appear to have greater effect, and twice-daily dosing offers no advantage over the same total dose given once daily.[1] No adjustment of dosage is typically necessary for advanced age, renal impairment, or hepatic dysfunction. For patients with possible depletion of intravascular volume (e.g., patients treated with diuretics), olmesartan should be initiated with caution; consideration should be given to use of a lower starting dose in such cases.[1] If blood pressure is not controlled by Benicar alone, a diuretic may be added. Benicar may be administered with other antihypertensive agents. Benicar may be administered with or without food.[1]
Preparations
Olmesartan is marketed worldwide by Daiichi Sankyo and in India by Abbott Healthcare Pvt. Ltd. under the trade name WinBP, Zydus Cadila under the trade name Olmy and by Ranbaxy Laboratories Ltd. under the trade name Olvance and in Canada by Schering-Plough as Olmetec. Benicar HCT is the brand name of a medication containing olmesartan medoxomil in combination with hydrochlorothiazide, a thiazide diuretic. Three dosage combinations are available: 20 mg or 40 mg of olmesartan medoxomil combined with 12.5 mg of hydrochlorothiazide, or 40 mg of olmesartan medoxomil combined with 25 mg of hydrochlorothiazide.
See also
References
- ^ a b c d RxList Inc. (5 July 2007). "Benicar (olmesartan medoxomil)". RxList Inc.. http://www.rxlist.com/benicar-drug.htm. Retrieved 22 July 2010.
- ^ http://www.drugs.com/fda/benicar-olmesartan-ongoing-safety-review-12946.html
- ^ "BENICAR Prescribing Information". http://www.benicar.com/pdf/prescribing_information.pdf. Retrieved 2011-01-20.
External links
- Daiichi-Sankyo Benicar page
- Benicar HCT from RXlist.com
Antihypertensives: agents acting on the renin-angiotensin system (C09) ACE inhibitors
("-pril")Sulfhydryl-containing: Captopril • Zofenopril
Dicarboxylate-containing: Enalapril# • Ramipril • Quinapril • Perindopril • Lisinopril (+HCT) • Benazepril
Phosphonate-containing: Fosinopril
Other/ungrouped: Alacepril • Cilazapril • Delapril • Imidapril • Moexipril • Rentiapril • Spirapril • Temocapril • TrandolaprilAIIRAs/
("-sartan")Azilsartan • Candesartan • Eprosartan • Irbesartan • Losartan • Olmesartan • Tasosartan§ • Telmisartan (+HCT) • Valsartan (+HCT)Renin inhibitors/
("-kiren")Categories:- Angiotensin II receptor antagonists
- Imidazoles
- Tetrazoles
- Carboxylate esters
- Alcohols
Wikimedia Foundation. 2010.
