- Alendronic acid
-
Alendronic acid 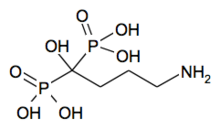
Systematic (IUPAC) name sodium [4-amino-1-hydroxy-1-(hydroxy-oxido-phosphoryl)- butyl]phosphonic acid trihydrate Clinical data Trade names Fosamax AHFS/Drugs.com monograph MedlinePlus a601011 Pregnancy cat. C Legal status ? Routes Oral Pharmacokinetic data Bioavailability 0.6% Metabolism excreted unchanged Half-life 126 months Excretion renal Identifiers CAS number 121268-17-5 
ATC code M05BA04 PubChem CID 2088 DrugBank APRD00561 ChemSpider 2004 
UNII X1J18R4W8P 
KEGG D07119 
ChEBI CHEBI:2567 
ChEMBL CHEMBL870 
Chemical data Formula C4H18NNaO10P2 Mol. mass 325.124 SMILES eMolecules & PubChem  (what is this?) acid (verify)
(what is this?) acid (verify)Alendronic acid (INN) or alendronate sodium (USAN) — sold as Fosamax by Merck — is a bisphosphonate drug used for osteoporosis and several other bone diseases. It is marketed alone as well as in combination with vitamin D (2,800 IU and 5600 IU, under the name Fosamax+D). Merck's U.S. patent on alendronate expired in 2008 and Merck lost a series of appeals to block a generic version of the drug from being certified by the U.S. Food and Drug Administration (FDA).
On February 6, 2008, the US FDA approved the first generic versions of alendronate, which were marketed by Barr Pharmaceuticals and Teva Pharmaceuticals USA. Teva Pharmaceuticals manufactures generic alendronate in 5-milligram, 10-milligram, and 40-milligram daily doses, and in 35-milligram and 70-milligram weekly doses, while Barr made generic alendronate in 70-milligram tablets, which were taken once weekly. [1]Barr pharmaceuticals were subsequently acquired by Teva in July 2008.
Contents
Pharmacokinetics
As with all potent bisphosphonates, the systemic bioavailability after oral dosing is low, averaging only 0.6–0.7% in women and in men under fasting conditions. Intake together with meals and beverages other than water further reduces the bioavailability. The absorbed drug rapidly partitions, with approximately 50% binding to the exposed bone surface; the remainder is excreted unchanged by the kidneys. Unlike most drugs, the strong negative charge on the two phosphonate moieties limits oral bioavailability, and, in turn, the exposure to tissues other than bone is very low. After absorption in the bone, alendronate has an estimated terminal half-life of 10 years.[2]
Pharmacology
Alendronate inhibits osteoclast-mediated bone-resorption. Like all bisphosphonates, it is chemically related to inorganic pyrophosphate, the endogenous regulator of bone turnover. Whereas pyrophosphate and the first bisphosphonate, etidronate, are capable of inhibiting both osteoclastic bone resorption as well as the mineralization of the bone newly formed by osteoblasts, alendronate and the other potent N-containing bisphosphonates such as risedronate, ibandronate, and zoledronate specifically inhibit bone resorption without any effect on mineralization at pharmacologically achievable doses. Its inhibition of bone-resorption is dose-dependent and approximately 1,000 times stronger than the equimolar effect of etidronate. Under therapy, normal bone tissue develops, and alendronate is deposited in the bone-matrix in pharmacologically inactive form. For optimal action, enough calcium and vitamin D are needed in the body in order to promote normal bone development. Hypocalcemia should, therefore, be corrected before starting therapy.
Clinical data
Treatment of post-menopausal women with Fosamax has demonstrated normalization of the rate of bone turnover, significant increase in BMD (bone mineral density) of the spine, hip, wrist and total body, and significant reductions in the risk of vertebral (spine) fractures, wrist fractures, hip fractures, and all non-vertebral fractures. In the women with the highest risk of fracture (by virtue of pre-existing vertebral fractures) in the Fracture Intervention Trial, treatment with Fosamax 5 mg/day for two years followed by 10 mg/day for the third year (plus calcium and vitamin D) resulted in approximately 50% reductions in fractures of the spine, hip, and wrist compared with the control group taking placebos plus calcium and vitamin D.[citation needed]
Uses
- Prophylaxis and treatment of female osteoporosis
- Treatment of male osteoporosis
- Prevention and treatment of corticosteroid-associated osteoporosis together with supplements of calcium and vitamin D
- Paget's disease
Contraindications and precautions
- Acute inflammations of the gastrointestinal tract (esophagitis, gastritis, ulcerations)
- Clinically manifest osteomalacia
- Certain malformations and malfunctions of the esophagus (strictures, achalasia)
- Inability to stand, walk, or sit for 30 minutes after oral administration
- Renal impairment with a creatinine clearance below 30ml/min
- Hypersensitivity to alendronate or another ingredient
- Hypocalcemia
- Pregnancy and breastfeeding
- Patients below 18 yrs. of age, as no clinical data exists
Side-effects
- Gastrointestinal tract: ulceration of the esophagus; this may require hospitalization and intensive treatment. Gastric and duodenal ulceration may also occur. December 31, 2008, the FDA reported alendronate and related drugs may carry an increased risk for esophageal cancer.[3]
- General: infrequent cases of skin rash, rarely manifesting as Stevens-Johnson syndrome and toxic epidermal necrolysis, eye problems (uveitis, scleritis) and generalized muscle, joint, and bone pain [4] (rarely severe) have been seen. In laboratory tests decreased calcium and phosphate values may be obtained but reflect action of the drug and are harmless.
- Osteonecrosis of the Jaw - Deterioration of the Temporomandibular Joint (TMJ) may occur while on this drug, if dental work of any kind is carried out.[5] Although osteonecrosis is uncommon, it occurs primarily in patients being administered intravenous biphosphonates, with most cases being reported in cancer patients.[citation needed]
- Neurological: Rare instances of auditory hallucinations and visual disturbances have been associated with alendronate and other bisphosphonates.[6]
- Bone: Alendronate has been linked in long-term users to the development of low-impact femoral fractures.[7] Further, studies suggest that users of alendronate have an increase in the numbers of osteoclasts and develop giant, more multinucleated osteoclasts; the significance of this development is unclear.[8] People who have taken Fosamax has been linked to a rare type of leg fracture that cuts straight across the upper thigh bone after little or no trauma. (Subtrochanteric fractures) [9] This is because Fosamax makes the thigh bone more brittle and stops the cells in the body that remodel the bone. Studies are showing that people who have taken Fosamax for more than five years are at risk for developing these kind of fractures. In some cases, patients have reported that, after weeks or months of unexplained aching, their thigh bones simply snapped while they were walking or standing. One doctor reports that a 59-year old previously healthy woman visiting New York City was riding a subway train one morning when the train jolted. She shifted all her weight to one leg, felt a bone snap, and fell to the floor of the train. An x-ray in a local emergency room revealed a comminuted spiral fracture involving the upper half of the right femur. She had been taking Fosamax for 7 years. [10] On Oct. 13, 2010 the Food and Drug Administration issued a warning about these fractures. [11]
Interactions
- Milk, diet, and drugs containing high amounts of calcium, magnesium or aluminium (antacids): the resorption of alendronate is decreased. At least half an hour should pass after intake of alendronate before taking the supplement or drug.
- Highly active vitamin D analogues or fluorides: no data is available. Concomitant treatment should be avoided.
- The additional beneficial effect of HRT (hormone replacement therapy) with estrogens/progestins or raloxifene in postmenopausal women remains to be elucidated, but no interactions have been seen. The combination is therefore possible.
- Intravenous ranitidine increases the oral bioavailability of alendronate. No clinical consequences are known.
- The combination of NSAIDs and alendroate may increase the risk of gastric ulcers. Both these drugs have the potential to irritate the upper gastro-intestinal mucosa.
Dosage
- Prophylaxis of osteoporosis in women: 5-10 mg daily or 35-70 mg weekly.
- Therapy of osteoporosis in women and men : 10 mg daily or 70 mg weekly.
- Osteoporosis under corticosteroids: 5-10 mg daily or 35-70 mg weekly in men and premenopausal women or those receiving concomitant HRT. In postmenopausal women not receiving HRT, the recommended dose is 10 mg daily or 70 mg weekly.
- Paget's Disease: 40 mg daily for 6 months.
The drug is to be taken only upon rising for the day with three swallows of water, not to exceed 6-8 oz. Stand, walk, or sit, and remain fasting for 30-45 minutes afterwards, then eat breakfast. Lying down or reclining after taking the drug and prior to eating breakfast may cause gastroesophageal reflux and esophageal irritation. At least 30 minutes should be allowed to pass before meals or other beverages than water are taken in.
On December 31, 2008, a letter in the New England Journal of Medicine cited a finding by the U.S. Food and Drug Administration that 23 cases of esophageal cancer, possibly linked to the use of the drug, have been seen since Fosamax's 1995 market debut.[12]
Dosage forms
- Fosamax solution 70 mg/75ml
- Fosamax tablets 5 mg, 10 mg, 35 mg, 40 mg, and 70 mg
Litigation
By 2000, the FDA had received 139 spontaneous reports through the MedWatch system of osteonecrosis of the jaw (ONJ) in cancer patients treated with intravenous (IV) bisphopshonates, either IV pamidronate (Aredia) or IV zoledronate (Zometa).
At the 2004 ODAC meeting the number of spontaneous reports had increased since 2000, and although almost all of the reports remained in cancer patients treated with one of the IV bisphosphonates 13 of the reports were in patients taking oral alendronate or risedronate, most of which were reported on September 24, 2004, in the Journal of Oral and Maxillofacial Surgery. Although this small number of reports does not remotely indicate causality, given the millions of patients who have been treated with alendronate and risedronate, in order to be cautious the FDA asked the manufacturers of the oral bisphosphonates to issue a warning to healthcare professionals of the potential that bisphosphonates might increase the risk of ONJ.
Despite the fact that no data links the oral bisphosphonates causally to ONJ, product liability attorneys immediately filed suit maintaining that their claimants' ONJ is a direct result of the use of alendronate. Merck has stated the "underlying cause" of osteonecrosis of the jaw is "uncertain", though it might be triggered by a traumatic event like tooth extraction or oral surgery. As of May 13, 2007, hundreds of cases had been filed against Merck alleging Fosamax-induced ONJ. The first case is set to be tried in late 2008.
Bis-phossy jaw
The term given by scientists to the link between bisphosphonates and jaw necrosis is 'bis-phossy jaw.' This is derived from the 19th-century term phossy jaw, given its name after workers in match factories working with white phosphorus developed osteonecrosis of the jaw.
References
- ^ "First Generic Fosamax OK'd by FDA". http://www.rxlist.com/script/main/art.asp?articlekey=87025. Retrieved 2008-02-21.
- ^ Shinkai, I. O. (Jan 1996). "New drugs--reports of new drugs recently approved by the FDA. Alendronate". Bioorganic & medicinal chemistry 4 (1): 3–4. doi:10.1016/0968-0896(96)00042-9. ISSN 0968-0896. PMID 8689235.
- ^ Gene Emery (Reuters) (December 31, 2008). "Esophageal cancer linked to osteoporosis drugs.". http://uk.reuters.com/article/healthNewsMolt/idUKTRE4BU4TX20081231. Retrieved March 9, 2010.
- ^ FDA Patient Safety News, March 2008
- ^ Fosamax product description, Merck & Co
- ^ Craig I. Coleman, Kristen A. Perkerson, Anne Lewis (July 2, 2004). "Alendronate-Induced Auditory Hallucinations and Visual Disturbances". http://www.medscape.com/viewarticle/481375_1. Retrieved March 9, 2010.
- ^ Serena Gordon (March 19, 2008). "Fosamax Linked to Unusual Femur Fractures.Osteoporosis drug also linked to bone pain and irregular heartbeats in past research". http://health.usnews.com/usnews/health/healthday/080319/fosamax-linked-to-unusual-femur-fractures.htm. Retrieved March 9, 2010.
- ^ Gardner, Amanda (December 31, 2008). "Osteoporosis Drug Prompts Increase in Certain Bone Cells". The Washington Post. http://www.washingtonpost.com/wp-dyn/content/article/2008/12/31/AR2008123102537.html.
- ^ Kwek, E; Goh, S; Koh, J; Png, M; Howe, T (2008). "An emerging pattern of subtrochanteric stress fractures: A long-term complication of alendronate therapy". Injury 39 (2): 224–231. doi:10.1016/j.injury.2007.08.036. PMID 18222447.
- ^ http://www.jenniferschneider.com/articles/An_unusual_case.html
- ^ http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm229171.htm
- ^ http://abcnews.go.com/Health/ActiveAging/story?id=6558069&page=1
External links
- Fosamax information from Merck
- RxList page
- MedlinePlus listing
- Pharmacotherapy 24(6):799-802, 2004
- Fosamax Overview @ FDA
Merck & Co., Inc. Corporate directors: Richard Clark · Johnnetta Cole · William Harrison · William Kelley · Rochelle Lazarus · Thomas Shenk · Anne Tatlock · Samuel Thier · Wendell Weeks · Peter WendellProducts: Alendronate · Aprepitant · Ertapenem · Ezetimibe · Ezetimibe/simvastatin · Finasteride · Fosaprepitant · Indinavir · Losartan · Lovastatin · Montelukast · Raltegravir · Rizatriptan · Rofecoxib · Simvastatin · Sitagliptin · VorinostatPublications: The Merck Manuals (Index · Manual · Veterinary · USD ( 2% FY 2004) · Employees: 63,000 · Stock Symbol: NYSE: MRK · Website: merck.com
2% FY 2004) · Employees: 63,000 · Stock Symbol: NYSE: MRK · Website: merck.comDrugs for treatment of bone diseases (M05) Bisphosphonates Nitrogenous (Alendronic acid, ibandronic acid, incadronic acid, pamidronic acid, risedronic acid, zoledronic acid)
Non-nitrogenous (Etidronic acid, clodronic acid, tiludronic acid)Bone morphogenetic proteins Other Resorption inhibitor (Ipriflavone) • Aluminium chlorohydrate • Dual action bone agent (Strontium ranelate) •
RANKL inhibitor (Denosumab) • Cathepsin K inhibitor (Odanacatib)Categories:- Bisphosphonates
- Merck
Wikimedia Foundation. 2010.

