- Osteoclast
-
Osteoclast 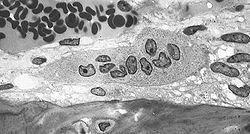
Osteoclast, with bone below it, showing typical distinguishing characteristics: a large cell with multiple nuclei and a "foamy" cytosol. Latin osteoclastus Code TH H2.00.03.7.00005 An osteoclast (from the Greek words for "bone" (Οστό) and "broken" (κλαστός)) is a type of bone cell that removes bone tissue by removing its mineralized matrix and breaking up the organic bone (organic dry weight is 90% collagen). This process is known as bone resorption. Osteoclasts were discovered by Kolliker in 1873.[1] Osteoclasts and osteoblasts are instrumental in controlling the amount of bone tissue: osteoblasts form bone, osteoclasts resorb bone. Osteoclasts are formed by the fusion of cells of the monocyte-macrophage cell line.[2] Osteoclasts are characterized by high expression of tartrate resistant acid phosphatase (TRAP) and cathepsin K.
Contents
Morphology
An osteoclast is a large cell that is 40 micrometer in diameter. They contain 15-20 closely packed oval-shaped nuclei. They are found in pits in the bone surface which are called resorption bays, or Howship's Lacunae. Osteoclasts are characterized by a cytoplasm with a homogeneous, "foamy" appearance. This appearance is due to a high concentration of vesicles and vacuoles. These vacuoles are lysosomes filled with acid phophatase. Osteoclast rough endoplasmic reticulum is sparse, and the Golgi complex is extensive.[3][4][5]
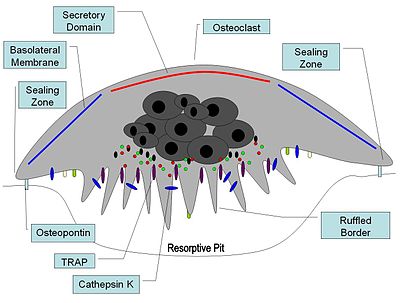
At a site of active bone resorption, the osteoclast forms a specialized cell membrane, the "ruffled border," that touches the surface of the bone tissue.[2] The ruffled border, which facilitates removal of the bony matrix, is a morphologic characteristic of an osteoclast that is actively resorbing bone. The ruffled border increases surface area interface for bone resorption. The mineral portion of the matrix (called hydroxyapatite) includes calcium and phosphate ions. These ions are absorbed into small vesicles (see endocytosis), which move across the cell and eventually are released into the extracellular fluid, thus increasing levels of the ions in the blood.
Origin
Since their discovery in 1873 there has been considerable debate about their origin. Three theories were dominant: from 1949 to 1970 the connective tissue origin was popular, which stated that osteoclasts and osteoblasts are of the same lineage, and ostoblasts fuse together to form osteoclasts. At certain times osteoclasts dissociate into osteoblasts, which finally form osteocytes. In the 1970s the biphyletic theory became popular; it states that osteoblasts and osteoclasts are of different lineage. It was in the beginning of 1980 that the monocyte phagocytic system was recognized as precursor of osteoclasts.[6] Osteoclast formation requires the presence of RANK ligand (receptor activator of nuclear factor κβ) and M-CSF (Macrophage colony-stimulating factor). These membrane bound proteins are produced by neighbouring stromal cells and osteoblasts, thus requiring direct contact between these cells and osteoclast precursors.
M-CSF acts through its receptor on the osteoclast, c-fms (colony-stimulating factor 1 receptor), a transmembrane tyrosine kinase-receptor, leading to secondary messenger activation of tyrosine kinase Src. Both of these molecules are necessary for osteoclastogenesis and are widely involved in the differentiation of monocyte/macrophage derived cells.
RANKL is a member of the tumour necrosis family (TNF), and is essential in osteoclastogenesis. RANKL knockout mice exhibit a phenotype of osteopetrosis and defects of tooth eruption, along with an absence or deficiency of osteoclasts. RANKL activates NF-κβ (nuclear factor-κβ) and NFATc1 (nuclear factor of activated t cells, cytoplasmic, calcineurin-dependent 1) through RANK. NF-κβ activation is stimulated almost immediately after RANKL-RANK interaction occurs, and is not upregulated. NFATc1 stimulation, however, begins ~24–48 hours after binding occurs and its expression has been shown to be RANKL dependent.
Osteoclast differentiation is inhibited by osteoprotegerin (OPG), which is produced by osteoblasts and binds to RANKL thereby preventing interaction with RANK.
Function
Once activated, osteoclasts move to areas of microfracture in the bone by chemotaxis. Osteoclasts lie in a small cavity called Howship's lacunae, formed from the digestion of the underlying bone. The sealing zone is the attachment of the osteoclast's plasmalemma to the underlying bone. Sealing zones are bounded by belts of specialized adhesion structures called podosomes. Attachment to the bone matrix is facilitated by integrin receptors, such as αvβ3, via the specific amino acid motif Arg-Gly-Asp in bone matrix proteins, such as osteopontin. The osteoclast releases hydrogen ions through the action of carbonic anhydrase (H2O + CO2 → HCO3- + H+) through the ruffled border into the resorptive cavity, acidifying and aiding dissolution of the mineralized bone matrix into Ca2+, H3PO4, H2CO3, water and other substances. Dysfunction of the carbonic anhydrase has been documented to cause some forms of osteopetrosis. Hydrogen ions are pumped against a high concentration gradient by proton pumps, specifically a unique vacuolar-ATPase. This enzyme has been targeted in the prevention of osteoporosis. In addition, several hydrolytic enzymes, such as members of the cathepsin and matrix metalloprotease(MMP) groups , are released to digest the organic components of the matrix. These enzymes are released into the compartment by lysosomes. Of these hydrolytic enzymes, cathepsin K is of most importance.
Cathepsin K and other cathepsins
Cathepsin K is a collagenolytic, papain-like, cysteine protease that is mainly expressed in osteoclasts, and is secreted into the resorptive pit. Mutations in the cathepsin K gene are associated with pycnodysostosis, a hereditary osteopetrotic disease, characterised by lack of functional cathepsin K expression. Knockout studies of cathepsin K in mice lead to an osteopetrotic phenotype, which, is partially compensated by increased expression of proteases other that cathepsin K and enhanced osteoclastogenesis.
Cathepsin K has an optimal enzymatic activity in acidic conditions. It is synthesized as a proenzyme with a molecular weight of 37kDa, and upon activation by autocatalytic cleavage, is transformed into the mature, active form with a molecular weight of ~27kDa.
In the osteoclast, cathepsin K functions in the resorptive process. Upon polarization of the osteoclast over the site of resorption, cathepsin K is secreted from the ruffled border into the resorptive pit. Here, it is the major protease involved in the degradation of type I collagen and other noncollagenous proteins, which have been demineralized by the acidic environment of the resorptive pit. From the resorptive pit, cathepsin K transmigrates across the ruffled border, through the osteoclast via intercellular vesicles and is then released by the functional secretory domain. Within these intercellular vesicles, cathepsin K, along with ROS generation by TRAP further degrades bone resorption products.
Numerous other cathepsins are expressed in osteoclasts. These include cathepsin B, C, D, E, G, and L. The function of these cysteine and aspartic proteases is generally unknown within bone, and they are expressed at much lower levels than cathepsin K.
Studies on cathepsin L knockout mice have been mixed, with a report of reduced trabecular bone in homozygous and heterozygous cathepsin L knockout mice compared to wild-type and another report finding no skeletal abnormalities.
Matrix metalloproteinases
The matrix metalloproteinases (MMPs) comprise a family of more than 20 zinc-dependent endopeptidases. The role of matrix metalloproteinases (MMPs) in osteoclast biology is ill-defined, but in other tissue they have been linked with tumor promoting activities, such as activation of growth factors and are required for tumor metastasis and angiogenesis.
MMP-9 is associated with the bone microenvironment. It is expressed by osteoclasts, and is known to be required for osteoclast migration and is a powerful gelatinase. Transgenic mice lacking MMP-9 develop defects in bone development, intraosseous angiogenesis, and fracture repair.
MMP-13 is believed to be involved in bone resorption and in osteoclast differentiation, as knockout mice revealed decreased osteoclast numbers, osteopetrosis, and decreased bone resorption.
MMPs expressed by the osteoclast include MMP-9, -10, -12, and -14. apart from MMP-9, little is known about their relevance to the osteoclast, however, high levels of MMP-14 are found at the sealing zone.
Regulation
Osteoclasts are regulated by several hormones, including parathyroid hormone (PTH) from the parathyroid gland, calcitonin from the thyroid gland, and growth factor interleukin 6 (IL-6). This last hormone, IL-6, is one of the factors in the disease osteoporosis, which is an imbalance between bone resorption and bone formation. Osteoclast activity is also mediated by the interaction of two molecules produced by osteoblasts, namely osteoprotegerin and RANK ligand. Note that these molecules also regulate differentiation of the osteoclast.[7]
Alternate use of term
An osteoclast can also be an instrument used to fracture and reset bones (the origin is Greek osteon:bone and klastos:broken). To avoid confusion, the cell was originally termed osotoclast. When the surgical instrument went out of use, the cell became known by its present name.
References
- ^ Nijweidi Peter J., JEAN H. M. FEYEN (1986). "Cells of Bone: Proliferation, Differentiation, and Hormonal Regulation". Physiological Reviews 66 (4): 855–886. PMID 3532144.
- ^ a b Netter, Frank H. (1987), Musculoskeletal system: anatomy, physiology, and metabolic disorders. Summit, New Jersey: Ciba-Geigy Corporation ISBN 0914168886, p. 169
- ^ Standring S., Ed. Gray's Anatomy. 39th ed. 2005, Elsevier
- ^ Holtrop, M. E. and G. J. King (1977) (1977). "The ultrastructure of the osteoclast and its functional implications". Clin Orthop Relat Res 123 (123): 177–196. PMID 856515.
- ^ Väänänen H, Zhao H, Mulari M, Halleen J (2000). "The cell biology of osteoclast function". J Cell Sci 113 ( Pt 3): 377–81. PMID 10639325. p. 378
- ^ Peter J. Nijweide Ehb And Jean H. M. Feyen. Cells of Bone: Proliferation, Differentiation, and Hormonal Regulation. Physiological Reviews 1986;66(4):855-886.
- ^ Schoppet M, Preissner K, Hofbauer L (2002). "RANK ligand and osteoprotegerin: paracrine regulators of bone metabolism and vascular function". Arterioscler Thromb Vasc Biol 22 (4): 549–53. doi:10.1161/01.ATV.0000012303.37971.DA. PMID 11950689.
External links
Myeloid lineage - Blood (WBC and RBC) Cellular/
HSCsCFU-GMHistiocytes · Kupffer cells · Alveolar macrophage · Microglia · Osteoclasts · Epithelioid cells · giant cells (Langhans giant cells, Foreign-body giant cell, Touton giant cells)CFU-DLCommonCFU-BasoCFU-EosCFU-MegCFU-ECFU-MastNoncellular Musculoskeletal system · connective tissue: bone and cartilage (TA A02.0, TH H3.01, GA 2.86–95) Cartilage perichondrium · fibrocartilage callus · metaphysis
cells (chondroblast · chondrocyte)
types (hyaline · elastic · fibrous)Bone CycleTypesRegionsStructureosteon / Haversian system · Haversian canals · Volkmann's canals · connective tissue (endosteum · periosteum) · Sharpey's fibres · enthesis · lacunae · canaliculi · trabeculae · medullary cavity · bone marrowShapesHuman cell types / list derived primarily from mesoderm Paraxial muscle: Myoblast → Myocyte · Myosatellite cell · Tendon cell · Cardiac muscle cell
adipose: Lipoblast → AdipocyteDigestive systemIntermediate Urinary system (RSC)Angioblast → Endothelial cell · Mesangial cell (Intraglomerular, Extraglomerular) · Juxtaglomerular cell · Macula densa cell
Stromal cell → Interstitial cell → Telocytes
Simple epithelial cell → Podocyte · Kidney proximal tubule brush border cellLateral plate/
hemangioblastsee lymphocytessee myeloid cellsCategories:- Blood cells
- Macrophages
- Cell biology
- Human cells
Wikimedia Foundation. 2010.