- Simvastatin
-
Simvastatin 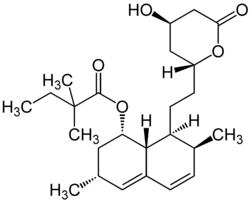

Systematic (IUPAC) name (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotetrahydro-2H-pyran-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate Clinical data Trade names Zocor AHFS/Drugs.com monograph MedlinePlus a692030 Licence data US FDA:link Pregnancy cat. D(AU) X(US) Legal status Prescription Only (S4) (AU) P (UK) ℞-only (US) Routes Oral Pharmacokinetic data Bioavailability 5% Protein binding 95% Metabolism Hepatic (CYP3A4) Half-life 2 hours for simvastatin and 1.9 hours for simvastatin acid Excretion Renal 13%, faecal 60% Identifiers CAS number 79902-63-9 
ATC code C10AA01 PubChem CID 54454 DrugBank APRD00104 ChemSpider 49179 
UNII AGG2FN16EV 
KEGG D00434 
ChEBI CHEBI:9150 
ChEMBL CHEMBL1064 
Chemical data Formula C25H38O5 Mol. mass 418.566 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Simvastatin (INN) (
 /ˈsɪmvəstætɨn/) (Zocor and generics) is a hypolipidemic drug used to control elevated cholesterol, or hypercholesterolemia. Simvastatin is a member of the statin class of pharmaceuticals, is a synthetic derivate of a fermentation product of Aspergillus terreus.
/ˈsɪmvəstætɨn/) (Zocor and generics) is a hypolipidemic drug used to control elevated cholesterol, or hypercholesterolemia. Simvastatin is a member of the statin class of pharmaceuticals, is a synthetic derivate of a fermentation product of Aspergillus terreus.Contents
Medical uses
The primary uses of simvastatin is for the treatment of dyslipidemia and the prevention of cardiovascular disease.[1] It is recommended to be used only after other measures such as diet, exercise, and weight reduction have not improved cholesterol levels.[1]
Adverse effects
Common side effects (>1% incidence) may include abdominal pain, diarrhea, indigestion, and a general feeling of weakness. Rare side effects include joint pain, memory loss, and muscle cramps.[2] Cholestatic hepatitis, hepatic cirrhosis, rhabdomyolysis and myositis have been reported in patients receiving the drug chronically.[3]
A type of DNA variant known as a single nucleotide polymorphism (SNP) may help predict individuals prone to developing myopathy when taking simvastatin; a study ultimately including 32,000 patients concluded that carriers of one or two risk alleles of particular SNPs rs4149056 were at 5x or 16x increased risk, respectively.[4]
On June 8, 2011, the FDA announced new safety recommendations for high-dose simvastatin (80 mg) due to muscle injury risk.[5][6]
Cost / benefit
Since its introduction, there has been a large debate surrounding the price for lipid-lowering treatment and its benefits with regard to atherosclerosis. Although this has affected the other statins, simvastatin was the first statin drug to be used extensively in clinical practice.
A number of large epidemiological studies were conducted to discover which patients would benefit most from statin drugs; most studies involve simvastatin as the study drug. The most influential studies were the Scandinavian Simvastatin Survival Study (4S) and the Heart protection study (HPS).
It has been suggested that patients with one or more risk factors for cardiovascular disease (such as diabetes mellitus, hypertension or a positive family history) can benefit from statins even if they do not have substantially elevated cholesterol levels.[citation needed]
Simvastatin was introduced in the late 1980s, and in many countries it is now available as a generic preparation. This has led to a decrease of the price of most statin drugs, and a reappraisal of the health economics of preventive statin treatment. In the UK in 2008 the typical per patient cost to the NHS of simvastatin was approx £1.50 per month.
Dose
Simvastatin is a powerful lipid-lowering drug that can decrease low density lipoprotein (LDL) levels by up to 50%. It is used in doses of 5 mg up to 80 mg. Higher doses (160 mg) have been found to be too toxic, while giving only minimal benefit in terms of lipid lowering.
In secondary prevention, 80 mg per day reduced major cardiovascular events by an absolute rate of 1.2% compared to 20 mg per day in a randomized controlled trial.[7]
Pharmacology and dosage
Main article: StatinAll statins act by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A HMG-CoA reductase, the rate-limiting enzyme of the HMG-CoA reductase pathway, the metabolic pathway responsible for the endogenous production of cholesterol. Statins are more effective than other lipid-regulating drugs at lowering LDL-cholesterol concentration but they are less effective than the fibrates in reducing triglyceride concentration. However, statins reduce cardiovascular disease events and total mortality irrespective of the initial cholesterol concentration.
The drug is in the form of an inactive lactone that is hydrolyzed after ingestion to produce the active agent. It is a white, nonhygroscopic, crystalline powder that is practically insoluble in water, and freely soluble in chloroform, methanol and ethanol.
Interactions
Grapefruit contains furanocoumarins, notably bergamottin and 6',7'-dihydroxybergamottin, which inhibit the intestinal cytochrome P450 3A4 isoform. This in turn slows metabolization of simvastatin and a large number of other drugs resulting in higher plasma levels of the drug. Due to the risk of toxicity patients taking simvastatin should avoid intake of grapefruit and grapefruit-containing products.[8]
On August 8, 2008, the U.S. Food and Drug Administration (FDA) issued a warning of the risk of rhabdomyolysis, which can lead to kidney failure or death, when simvastatin is used with amiodarone. This interaction is dose-dependent with simvastatin doses exceeding 20 mg. This drug combination especially with higher doses of simvastatin should be avoided.[9]
On March 19, 2010 the FDA issued another statement regarding simvastatin that said it increases the risk of muscle injury (myopathy) when taken at high doses (80 mg) or at lower doses in combination with other drugs. This conclusion is supported by the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) trial.[10] The highest 80 mg dose rate causes muscle damage in 61 out of every 1,000 patients, according to the research, in contrast to the lower 40 mg dose which causes muscle damage in 8 out of every 10,000 patients.[11] The FDA warning, re-released on June 8, 2011, suggested that "Simvastatin 80 mg should be used only in patients who have been taking this dose for 12 months or more without evidence of muscle injury (myopathy)" and that "Simvastatin 80 mg should not be started in new patients, including patients already taking lower doses of the drug."[12]
Also, a 2010 FDA review of Simvastatin drug-drug interactions stated that patients should not take a simvastatin with Itraconazole, Ketoconazole, Erythromycin, Clarithromycin, Telithromycin, HIV protease inhibitors, or Nefazodone. Patients taking 10 mg of simvastatin should not take it with Gemfibrozil, Cyclosporine, or Danazol. If taking 20 mg of simvastatin, do not take it with Amiodarone or Verapamil. Diltiazem should not be taken with 40 mg of simvastatin.[10] These drugs mentioned are CYP3A4 inhibitors which decrease the metabolism of simvastatin, therefore increasing the plasma activity of simvastatin which leads to higher risk of developing rhabdomyolysis and myopathy.[13]
Simvastatin is contraindicated in pregnancy and liver disease.[13]
Marketing
Reference: Drug Discovery Today editorial, 2005.[14]
Simvastatin was initially marketed by Merck & Co under the trade name Zocor, but is available generically in most countries following the patent expiry. A combination of simvastatin along with ezetimibe is sold under the brand name Vytorin and is jointly marketed by Merck and Schering-Plough.
Brand names include Zocor, Zocor Heart Pro, marketed by the pharmaceutical company Merck & Co. Simlup, Simvotin, Simcard [India], Denan (Germany), Liponorm, Sinvacor, Sivastin (Italy), Lipovas (Japan), Lodales (France), Zocord (Austria and Sweden), Zimstat, Simvahexal (Australia), Lipex (Australia and New Zealand), Simvastatin-Teva, Simvacor, Simvaxon, Simovil (Israel), and others.
The primary U.S. patent for Zocor expired on June 23, 2006.[citation needed] Ranbaxy Laboratories (at the 80 mg strength) and Teva Pharmaceutical Industries through its Ivax Pharmaceuticals unit (at all other strengths) were given approval by the FDA to manufacture and sell simvastatin as a generic drug with 180-day exclusivity. Dr. Reddy's Laboratories also has a license from Merck & Co. to sell simvastatin as an authorized generic drug.
Sales
Prior to losing U.S. patent protection, simvastatin was Merck & Co.'s largest selling drug and second largest selling cholesterol lowering drug in the world; it recorded US$4.3 billion of sales in 2005.[citation needed]
Zocor had an original patent expiry date of January 2006 but was extended by the United States Patent Trademark Office (PTO) to expire on June 23, 2006. The PTO granted the patent extension after Merck submitted data from studies of the drug’s positive effect on children, a move typically used by drug companies to lengthen exclusivity. In the UK the patent for simvastatin had expired by 2004.[citation needed]
Ordinarily, Merck would have expected a sharp decrease in sales after the generic versions of simvastatin entered the market. However, Merck has slashed the price of Zocor dramatically in an effort to claim sales that would have otherwise gone to the generic versions. At least two major U.S. health insurers, UnitedHealthcare and WellPoint, are now offering Zocor to their members as generic copays.[15]
In addition, since Merck manufactures some versions of Dr. Reddy's authorized generic simvastatin,[citation needed] Merck is poised to profit from Dr. Reddy's version. An 80 mg, 30-count bottle of Dr. Reddy's simvastatin obtained July 6, 2006 states it is made by Merck Sharp & Dohme (Merck & Co.'s name outside the U.S. to avoid conflicts with Merck KGaA) in the UK, just like 80 mg Zocor, and has a Merck & Co. logo on the bottom; except for omitting the "80" on one side, the tablets are visually identical to 80 mg Zocor, including "543" on the other side which is the key part of the National Drug Code for 80 mg Zocor.[citation needed]
History
The development of simvastatin was closely linked with lovastatin. Biochemist Jesse Huff and his colleagues at Merck began researching the biosynthesis of cholesterol in the early 1950s. In 1956, mevalonic acid was isolated from a yeast extract by Karl Folkers, Carl Hoffman, and others at Merck; while Huff and his associates confirmed that mevalonic acid was an intermediate in cholesterol biosynthesis. In 1959, the HMG-CoA reductase enzyme (a major contributor of internal cholesterol production) was discovered by researchers at the Max Planck Institute. This discovery encouraged scientists worldwide to find an effective inhibitor of this enzyme.
By 1976, Akira Endo had isolated the first inhibitor (Compactin, ML-236B) from the fungus Penicillium citrinium in Sankyo, Japan.[16] In 1979, Hoffman and colleagues isolated lovastatin from a strain of the fungus Aspergillus terreus. While developing and researching lovastatin, Merck scientists synthetically derived a more potent HMG-CoA reductase inhibitor from a fermentation product of Aspergillus terreus, which was designated MK-733 (later to be named simvastatin).[17]
See also
References
- ^ a b "Simvastatin". The American Society of Health-System Pharmacists. http://www.drugs.com/monograph/simvastatin.html. Retrieved 3 April 2011.
- ^ "Gen-Simvastatin - Drug Factsheets - C-Health". http://chealth.canoe.ca/drug_info_details.asp?channel_id=0&relation_id=0&brand_name_id=3499&page_no=. Retrieved 2007-08-15.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1431-1433.
- ^ SEARCH Collaborative Group,, Group; Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R (2008). "SLCO1B1 variants and statin-induced myopathy--a genomewide study". N Engl J Med. 359 (8): 789–99. doi:10.1056/NEJMoa0801936. PMID 18650507.
- ^ "FDA announces new safety recommendations for high-dose simvastatin" (Press release). U.S. Food and Drug Administration (FDA). http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm258338.htm. Retrieved 9 June 2011.
- ^ "FDA Restricts Use of High Doses of Cholesterol-Lowering Drug Zocor". Time. June 9, 2011.
- ^ Study Of The Effectiveness Of Add; Armitage, J; Bowman, L; Wallendszus, K; Bulbulia, R; Rahimi, K; Haynes, R; Parish, S et al. (2010). "Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12 064 survivors of myocardial infarction: a double-blind randomised trial". Lancet 376 (9753): 1658–69. doi:10.1016/S0140-6736(10)60310-8. PMC 2988223. PMID 21067805. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2988223.
- ^ National electronic library for medicines
- ^ "Information on Simvastatin/Amiodarone". http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm118869.htm. Retrieved 2008-09-21.
- ^ a b http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm
- ^ Steve Sternberg (2011-06-09). "Simvastatin can damage muscles in high doses". USA TODAY. http://yourlife.usatoday.com/health/medical/story/2011/06/Simvastatin-can-damage-muscles-in-high-doses/48208588/1. Retrieved 2011-06-09. "The cholesterol-lowering drug simvastatin can cause severe muscle damage and should not be prescribed in high doses to patients who have taken it for less than a year or in any dose to people taking certain drugs, health officials said Tuesday. ... Research has shown that the highest dose of simvastatin, 80 milligrams, causes muscle damage in 61 of every 1,000 patients, far higher than the eight-per-10,000 rate in patients taking a 40-milligram dose, Rosenblatt says."
- ^ "FDA Drug Safety Communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury". U.S. Food and Drug Administration. June 8, 2011. http://www.fda.gov/Drugs/DrugSafety/ucm256581.htm. Retrieved 2011-06-09. "The U.S. Food and Drug Administration (FDA) is recommending limiting the use of the highest approved dose of the cholesterol-lowering medication, simvastatin (80 mg) because of increased risk of muscle damage."
- ^ a b http://www.rxlist.com/zocor-drug.htm
- ^ Maggon K (June 2005). "Best-selling human medicines 2002-2004". Drug Discov. Today 10 (11): 739–42. doi:10.1016/S1359-6446(05)03468-9. PMID 15922927.
- ^ Brin, Dinah Wisenberg (2006-06-22). "Zocor Patent Expiring Means Bidding War". Associated Press. http://biz.yahoo.com/ap/060622/generic_drugs_zocor.html. Retrieved 2006-07-09.[dead link]
- ^ Liao JK, Laufs U (2005). "PLEIOTROPIC EFFECTS OF STATINS". Annu. Rev. Pharmacol. Toxicol. 45: 89–118. doi:10.1146/annurev.pharmtox.45.120403.095748. PMC 2694580. PMID 15822172. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2694580.
- ^ Olivia Williams, Anne-Marie Jacks, Jim Davis, Sabrina Martinez (1998). "Case 10: Merck(A): Mevacor*". In Allan Afuah. Innovation Management - Strategies, Implementation, and Profits. Oxford University Press. ISBN 0195113462. http://www-personal.umich.edu/~afuah/cases/case10.html. Retrieved 2006-07-19.
External links
Lipid modifying agents (C10) GI tract Ezetimibe • SCH-48461Liver Simvastatin# • Atorvastatin • Fluvastatin • Lovastatin • Mevastatin • Pitavastatin • Pravastatin • Rosuvastatin • Cerivastatin‡Blood vessels CETP inhibitors (HDL)Combinations Other Dextrothyroxine • Probucol • Tiadenol • Benfluorex • Meglutol • Omega-3-triglycerides • Magnesium pyridoxal 5-phosphate glutamate • Policosanol • Lapaquistat§ • Alipogene tiparvovecMerck & Co., Inc. Corporate directors: Richard Clark · Johnnetta Cole · William Harrison · William Kelley · Rochelle Lazarus · Thomas Shenk · Anne Tatlock · Samuel Thier · Wendell Weeks · Peter WendellProducts: Alendronate · Aprepitant · Ertapenem · Ezetimibe · Ezetimibe/simvastatin · Finasteride · Fosaprepitant · Indinavir · Losartan · Lovastatin · Montelukast · Raltegravir · Rizatriptan · Rofecoxib · Simvastatin · Sitagliptin · VorinostatPublications: The Merck Manuals (Index · Manual · Veterinary · USD ( 2% FY 2004) · Employees: 63,000 · Stock Symbol: NYSE: MRK · Website: merck.comCategories:
2% FY 2004) · Employees: 63,000 · Stock Symbol: NYSE: MRK · Website: merck.comCategories:- Alcohols
- Carboxylate esters
- Lactones
- Merck
- Statins
- Tetrahydropyrans
- World Health Organization essential medicines
Wikimedia Foundation. 2010.

