- Pravastatin
-
Pravastatin 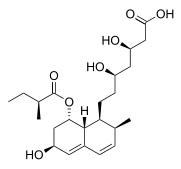
Systematic (IUPAC) name (3R,5R)-3,5-dihydroxy-7-((1R,2S,6S,8R,8aR)-6-hydroxy-2-methyl-8-{[(2S)-2-methylbutanoyl]oxy}-1,2,6,7,8,8a-hexahydronaphthalen-1-yl)-heptanoic acid Clinical data Trade names Pravachol AHFS/Drugs.com monograph MedlinePlus a692025 Pregnancy cat. X Legal status ? Routes oral Pharmacokinetic data Bioavailability 17% Protein binding 50% Metabolism renal and hepatic Half-life 1.5-2 hours Identifiers CAS number 81093-37-0 
ATC code C10AA03 PubChem CID 54687 DrugBank APRD00328 ChemSpider 49398 
UNII KXO2KT9N0G 
ChEMBL CHEMBL1144 
Chemical data Formula C23H36O7 Mol. mass 424.528 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Pravastatin (marketed as Pravachol or Selektine) is a member of the drug class of statins, used for lowering cholesterol and preventing cardiovascular disease. Initially known as CS-514, it was originally identified in a bacterium called Nocardia autotrophica by researchers of the Sankyo Pharma Inc..[1] It is presently being marketed outside Japan by the pharmaceutical company Bristol-Myers Squibb.
The U.S. Food and Drug Administration approved generic pravastatin for sale in the United States for the first time on April 24, 2006. Generic pravastatin sodium tablets are manufactured by TEVA Pharmaceuticals in Kfar Sava, Israel.[2]
Health effects
The primary uses of prevastatin is for the treatment of dyslipidemia and the prevention of cardiovascular disease.[3] It is recommended to be used only after other measures such as diet, exercise, and weight reduction have not improved cholesterol levels.[3]
References
- ^ Yoshino G, Kazumi T, Kasama T, et al. (1986). "Effect of CS-514, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, on lipoprotein and apolipoprotein in plasma of hypercholesterolemic diabetics". Diabetes Res. Clin. Pract. 2 (3): 179–81. doi:10.1016/S0168-8227(86)80020-1. PMID 3091343.
- ^ "FDA Approves First Generic Pravastatin". http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108644.htm. Retrieved 2008-01-20.
- ^ a b "Prevachol". The American Society of Health-System Pharmacists. http://www.drugs.com/monograph/pravachol.html. Retrieved 3 April 2011.
External links
Lipid modifying agents (C10) GI tract Ezetimibe • SCH-48461Liver Simvastatin# • Atorvastatin • Fluvastatin • Lovastatin • Mevastatin • Pitavastatin • Pravastatin • Rosuvastatin • Cerivastatin‡Blood vessels CETP inhibitors (HDL)Combinations Other Dextrothyroxine • Probucol • Tiadenol • Benfluorex • Meglutol • Omega-3-triglycerides • Magnesium pyridoxal 5-phosphate glutamate • Policosanol • Lapaquistat§ • Alipogene tiparvovec
This drug article relating to the cardiovascular system is a stub. You can help Wikipedia by expanding it.
