- Montelukast
-
Montelukast 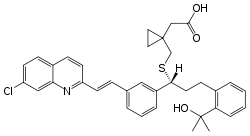
Systematic (IUPAC) name (S,E)-2-(1-((1-(3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-(2-(2-hydroxypropan-2-yl)phenyl)propylthio)methyl)cyclopropyl)acetic acid Clinical data Trade names Singulair AHFS/Drugs.com monograph MedlinePlus a600014 Pregnancy cat. B (U.S.), B1 (Au) Legal status Rx Only (U.S.), POM (UK), S4 (Au) Routes Oral Pharmacokinetic data Bioavailability 63% to 73% Protein binding 99% Metabolism Hepatic (CYP3A4 and CYP2C9-mediated) Half-life 2.7-5.5 hours Excretion Biliary Identifiers CAS number 158966-92-8 
ATC code R03DC03 PubChem CID 5281040 DrugBank APRD00434 ChemSpider 4444507 
UNII MHM278SD3E 
KEGG D08229 
ChEBI CHEBI:50730 
ChEMBL CHEMBL787 
Chemical data Formula C35H36ClNO3S Mol. mass 586.184 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Montelukast (trade names Singulair and Montelo-10) is a leukotriene receptor antagonist (LTRA) used for the maintenance treatment of asthma and to relieve symptoms of seasonal allergies.[1][2] It is usually administered orally. Montelukast is a CysLT1 antagonist; that it blocks the action of leukotriene D4 (and secondary ligands LTC4 and LTE4) on the cysteinyl leukotriene receptor CysLT1 in the lungs and bronchial tubes by binding to it. This reduces the bronchoconstriction otherwise caused by the leukotriene and results in less inflammation.
Because of its method of operation, it is not useful for the treatment of acute asthma attacks. Again because of its very specific focus of operation, it does not interact with other asthma medications such as theophylline.
Another leukotriene receptor antagonist is zafirlukast (Accolate), taken twice daily. Zileuton (Zyflo), an asthma drug taken four times per day, blocks leukotriene synthesis by inhibiting 5-lipoxygenase, an enzyme of the eicosanoid synthesis pathway.
The Mont in Montelukast stands for Montreal, the place where Merck developed the drug.[3]
Contents
Medical uses
Montelukast is used for a number of conditions including: asthma, exercise induced bronchospasm, allergic rhinitis, and urticaria.[4] It is mainly used as a complementary therapy in adults in addition to inhaled corticosteroids, if they alone do not bring the desired effect. Corticosteroids reduce inflammation but have no effect on leukotrienes.
Adverse effects
Side effects include gastrointestinal disturbances, hypersensitivity reactions, sleep disorders and increased bleeding tendency, aside from many other generic adverse reactions. Its use is associated with a higher incidence of Churg–Strauss syndrome (whether or not this drug is 'unmasking' subclinical Churg–Strauss is as yet uncertain).
FDA Investigation
In March, 2008 the FDA announced that it would investigate whether mood changes and suicidal thoughts are possible side effects of drugs in this class, including the popular drug Singulair, which currently lists these side effects.[5]
On June 12, 2009 the Food and Drug Administration concluded their review into the possibility of neuropsychiatric side effects with leukotriene modulator drugs. Although clinical trials only revealed an increased risk of insomnia, post-marketing surveillance showed that the drugs are associated with a possible increase in suicidal behavior and other side effects such as agitation, aggression, anxiousness, dream abnormalities and hallucinations, depression, irritability, restlessness and tremor.[6]
Use with loratadine
Schering-Plough and Merck have sought permission to market a combined tablet with loratadine (Claritin) and montelukast (Singulair), as many patients combine the two themselves. However, the FDA has found no benefit from a combined pill for seasonal allergies over taking the two drugs in combination,[7] and on April 25, 2008, issued a "not approvable" letter for the combination.[8]
Patents
Singulair is covered by U.S. Patent No. 5,565,473[9] which is set to expire (by pediatric extension) on August 3, 2012.[10]
On May 28, 2009, the U.S. Patent and Trademark Office announced their decision to launch a reexamination of the patent covering Singulair. The decision to reexamine was driven by the discovery of references that were not included in the original patent application process. The references were submitted through Article One Partners, an online research community focused on finding literature relating to existing patents. The references included a scientific article produced by a Merck employee around the key ingredient of Singulair, and a previously filed patent in the same technology area.[11]
On December 17, 2009, the U.S. Patent and Trademark Office determined that the patent in question was valid based on the initial reexamination and new information provided.[12]
References
- ^ Lipkowitz, Myron A. and Navarra, Tova (2001) The Encyclopedia of Allergies (2nd ed.) Facts on File, New York, p. 178, ISBN 0-8160-4404-X
- ^ "Asthma / Allergy ". Mascothealth.com. Retrieved 9 April 2011.
- ^ http://www.merckfrosst.ca/mfcl/en/corporate/research/accomplishments/singulair.html
- ^ "Montelukast Sodium". The American Society of Health-System Pharmacists. http://www.drugs.com/monograph/montelukast-sodium.html. Retrieved 3 April 2011.
- ^ FDA Investigates Merck Drug-Suicide Link
- ^ Updated Information on Leukotriene Inhibitors: Montelukast (marketed as Singulair), Zafirlukast (marketed as Accolate), and Zileuton (marketed as Zyflo and Zyflo CR). Food and Drug Administration. Published June 12, 2009. Accessed June 13, 2009.
- ^ Rubenstein, Sarah (April 28, 2008). "FDA Sneezes at Claritin-Singulair Combo Pill". The Wall Street Journal. http://blogs.wsj.com/health/2008/04/28/fda-sneezes-at-claritin-singulair-combo-pill/?mod=WSJBlog.
- ^ Schering-Plough press release - Schering-Plough/MERCK Pharmaceuticals Receives Not-Approvable Letter from FDA for Loratadine/Montelukast
- ^ 5,565,473
- ^ Singular patent details
- ^ "U.S. Reexamines Merck's Singulair Patent". Thompson Reuters. May 28, 2009. http://www.reuters.com/article/2009/05/28/merck-singulair-idUSN2834535820090528.
- ^ "Merck Says U.S. Agency Upholds Singulair Patent". Thompson Reuters. December 17, 2009. http://www.reuters.com/article/2009/12/17/merck-singulair-idUSWEN770820091217.
External links
Drugs for obstructive airway diseases: asthma/COPD (R03) Adrenergics, inhalants Salbutamol#/Levosalbutamol • Fenoterol • Terbutaline • Pirbuterol • Procaterol • Bitolterol • Rimiterol • Carbuterol • Tulobuterol • ReproterolLong acting β2-agonists (LABA)otherGlucocorticoids Anticholinergics/
muscarinic antagonistMast cell stabilizers Cromoglicate • NedocromilXanthines Eicosanoid inhibition Thromboxane receptor antagonistsCombination products Hypothalamic/pituitary axes Eicosanoid Other Merck & Co., Inc. Corporate directors: Richard Clark · Johnnetta Cole · William Harrison · William Kelley · Rochelle Lazarus · Thomas Shenk · Anne Tatlock · Samuel Thier · Wendell Weeks · Peter WendellProducts: Alendronate · Aprepitant · Ertapenem · Ezetimibe · Ezetimibe/simvastatin · Finasteride · Fosaprepitant · Indinavir · Losartan · Lovastatin · Montelukast · Raltegravir · Rizatriptan · Rofecoxib · Simvastatin · Sitagliptin · VorinostatPublications: The Merck Manuals (Index · Manual · Veterinary · USD ( 2% FY 2004) · Employees: 63,000 · Stock Symbol: NYSE: MRK · Website: merck.comCategories:
2% FY 2004) · Employees: 63,000 · Stock Symbol: NYSE: MRK · Website: merck.comCategories:- Leukotriene antagonists
- Merck
- Quinolines
- Organochlorides
- Alkenes
- Thioethers
- Cyclopropanes
Wikimedia Foundation. 2010.
