- Ciclesonide
-
Ciclesonide 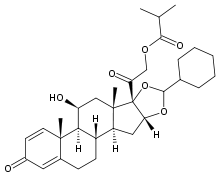
Systematic (IUPAC) name 2-[(1S, 2S, 4R, 8S, 9S,11S, 12S, 13R)-6-cyclohexyl-11-hydroxy-9, 13-dimethyl-16-oxo-5, 7-dioxapentacyclo [10.8.0.02,9.04, 8.013,18] icosa-14, 17-dien-8-yl]- 2-oxoethyl 2-methylpropanoate Clinical data AHFS/Drugs.com monograph MedlinePlus a607008 Pregnancy cat. B3(AU) C(US) Legal status Prescription Only (S4) (AU) POM (UK) ℞-only (US) Identifiers CAS number 141845-82-1 
ATC code R03BA08 PubChem CID 6918155 DrugBank DB01410 ChemSpider 5293368 
UNII S59502J185 
KEGG D01703 
ChEMBL CHEMBL1201164 
Synonyms (11β, 16α)-16, 17-[[(R)-cyclohexylmethylene]bis(oxy)]-11-hydroxy-21- (2-methyl-1-oxopropoxy)- pregna-1, 4-diene-3, 20-dione Chemical data Formula C32H44O7 Mol. mass 540.688 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Ciclesonide is a glucocorticoid used to treat obstructive airway diseases. It is marketed under the brand name Alvesco for asthma and Omnaris/Omniair for hayfever in the US & Canada. Phase 3 trials for the hayfever indication outside the US are ongoing.[1]
"A novel approach to minimizing the risk of toxicity from systemic absorption of an inhaled corticosteroid underlay the development of ciclesonide. This corticosteroid is inhaled as a prodrug activated by cleavage by esterases in bronchial epithelial cells. When absorbed into the circulation, the active product is tightly bound to serum proteins, and so has little access to glucocorticoid receptors in skin, eye, and bone, minimizing its risk of causing cutaneous thinning, cataracts, osteoporosis, or temporary slowing of growth" [2]
Contents
Indications
Maintenance treatment in persistent asthma; hayfever.
Specific Considerations
Pregnancy
No data as yet exists, Other inhaled glucocorticoids are considered ADEC category B3.
Breastfeeding
Should be safe to use. Consult your medical professional.[citation needed]
Practice Points
- Appears to be as effective as budesonide or fluticasone for maintenance treatment in persistent asthma although long term data on clinical outcomes are lacking.
- In short term studies ciclesonide had a minimal effect on markers of adrenal suppression but data with long term use is lacking. The US Food and Drug Administration (FDA) announced October 2006 the approval of ciclesonide nasal spray for the treatment of nasal symptoms associated with seasonal and perennial allergic rhinitis in adults and children 12 years of age and older.[3]
The safety and efficacy of the nasal spray, manufactured by ALTANA Pharma US, Inc. of Florham Park, NJ, were studied in randomized placebo controlled clinical trials. The studies showed that patients treated with nasal spray had an 8-10 percent greater reduction in nasal symptoms compared to placebo, with difference between ciclesonide nasal spray and placebo significant, i.e. p<0.05.
The highest level of conversion was found in the liver, the site of inactivation of des-CIC through rapid oxidation by cytochrome P450. Carboxylesterases in bronchial epithelial cells probably contribute significantly to the conversion to des-CIC in the target organ, whereas low systemic levels of des-CIC are a result of the high metabolic clearance by the liver following CIC inhalation.
The most common side effects were headache, nosebleeds, and inflammation of the nose and throat linings.[4]
References
- ^ "OMNARIS/OMNAIR". Products. Nycomed International Management GmbH. 2009. http://www.nycomed.com/Nycomed.MCms/Templates/Nycomed/Products/Products.aspx?NRMODE=Published&NRNODEGUID=%7b95B0E8B9-D1B3-4D3D-8B77-26AE2795B0F9%7d&NRORIGINALURL=%2fen%2fMenu%2fProducts%2fProduct%2boverview&NRCACHEHINT=Guest. Retrieved 2009-07-30.[dead link]
- ^ Katzung, BG. Basic and Clinical Pharmacology. 10th Ed. 2007. p324.
- ^ "FDA NEWS RELEASE. FDA Approves New Treatment for Allergies.". Food and Drug Administration. 2006-10-23. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108773.htm. Retrieved 2009-07-30.
- ^ Mutch, E; Nave R, McCracken N, Zech K, Williams FM (May 2007). "The role of esterases in the metabolism of ciclesonide to desisobutyryl-ciclesonide in human tissue.". Biochem Pharmacol. (England: Elsevier Science) 73 (10): 1657–1664. doi:10.1016/j.bcp.2007.01.031. ISSN 0006-2952. PMID 17331475. http://www.ncbi.nlm.nih.gov/pubmed/17331475?dopt=AbstractPlus. Retrieved 2007-07-30.
- Rossi S (Ed.) (2006). Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook. ISBN 0-9757919-2-3
Drugs for obstructive airway diseases: asthma/COPD (R03) Adrenergics, inhalants Salbutamol#/Levosalbutamol • Fenoterol • Terbutaline • Pirbuterol • Procaterol • Bitolterol • Rimiterol • Carbuterol • Tulobuterol • ReproterolLong acting β2-agonists (LABA)otherGlucocorticoids Beclometasone# • Budesonide • Ciclesonide • Fluticasone • Mometasone • Flunisolide • Betamethasone • TriamcinoloneAnticholinergics/
muscarinic antagonistMast cell stabilizers Cromoglicate • NedocromilXanthines Eicosanoid inhibition Thromboxane receptor antagonistsCombination products #WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III Decongestants and other nasal preparations (R01) Topical Sympathomimetics, plainCyclopentamine • Ephedrine • Phenylephrine • Oxymetazoline • Tetryzoline • Xylometazoline • Naphazoline • Tramazoline • Metizoline • Tuaminoheptane • Fenoxazoline • Tymazoline • EpinephrineSpaglumic acid
histamine antagonists (Levocabastine, Antazoline, Thonzylamine)
mast cell stabilizer (some are also antihistamines) (Cromoglicic acid, Nedocromil, Azelastine, Olopatadine, Lodoxamide)Beclometasone • Prednisolone • Dexamethasone • Flunisolide • Budesonide • Betamethasone • Tixocortol • Fluticasone • Mometasone furoate • Triamcinolone • CiclesonideOther nasal preparationsCafaminol • Calcium hexamine thiocyanate • Retinol • Ipratropium bromide • Ritiometan • Mupirocin • Hexamidine • Framycetin • Hyaluronic acid • Eucalyptus oilSystemic use:
Sympathomimetics
This drug article relating to the respiratory system is a stub. You can help Wikipedia by expanding it.
