- Corticosteroid
-
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex. Corticosteroids are involved in a wide range of physiologic systems such as stress response, immune response and regulation of inflammation, carbohydrate metabolism, protein catabolism, blood electrolyte levels, and behavior.
- Glucocorticoids such as cortisol control carbohydrate, fat and protein metabolism and are anti-inflammatory by preventing phospholipid release, decreasing eosinophil action and a number of other mechanisms.
- Mineralocorticoids such as aldosterone control electrolyte and water levels, mainly by promoting sodium retention in the kidney.
Some common natural hormones are corticosterone (C21H30O4), cortisone (C21H28O5, 17-hydroxy-11-dehydrocorticosterone) and aldosterone.
Contents
Biosynthesis
The corticosteroids are synthesized from cholesterol within the adrenal cortex. Most steroidogenic reactions are catalysed by enzymes of the cytochrome P450 family. They are located within the mitochondria and require adrenodoxin as a cofactor (except 21-hydroxylase and 17α-hydroxylase).
Aldosterone and corticosterone share the first part of their biosynthetic pathway. The last part is mediated either by the aldosterone synthase (for aldosterone) or by the 11β-hydroxylase (for corticosterone). These enzymes are nearly identical (they share 11β-hydroxylation and 18-hydroxylation functions), but aldosterone synthase is also able to perform an 18-oxidation. Moreover, aldosterone synthase is found within the zona glomerulosa at the outer edge of the adrenal cortex; 11β-hydroxylase is found in the zona fasciculata and zona glomerulosa.
Classification
By chemical structure
In general, corticosteroids are grouped into four classes, based on chemical structure. Allergic reactions to one member of a class typically indicate an intolerance of all members of the class. This is known as the "Coopman classification",[1] after S. Coopman, who defined this classification in 1989.[2]
The highlighted steroids are often used in the screening of allergies to topical steroids.[3]
Group A - Hydrocortisone Type
Hydrocortisone, hydrocortisone acetate, cortisone acetate, tixocortol pivalate, prednisolone, methylprednisolone, and prednisone (Short- to medium-acting glucocorticoids).
Group B - Acetonides
Triamcinolone acetonide, triamcinolone alcohol, mometasone, amcinonide, budesonide, desonide, fluocinonide, fluocinolone acetonide, and halcinonide.
Group C - Betamethasone Type
Betamethasone, betamethasone sodium phosphate, dexamethasone, dexamethasone sodium phosphate, and fluocortolone.
Group D - Esters
Group D1 - Halogenated (Less Labile)
hydrocortisone-17-valerate, aclometasone dipropionate, betamethasone valerate, betamethasone dipropionate, prednicarbate, clobetasone-17-butyrate, clobetasol-17-propionate, fluocortolone caproate, fluocortolone pivalate, and fluprednidene acetate.
Group D2 - Labile Prodrug Esters
Hydrocortisone-17-butyrate, 17-aceponate, 17-buteprate, and Prednicarbate.
By route of administration
Topical steroids
Main article: Topical steroidFor use topically on the skin, eye, and mucous membranes.
Topical corticosteroids are divided in potency classes I to IV, with the additional complication that in Europe class IV is the most potent, while in the US this is called Class I.
Inhaled steroids
for use to treat the nasal mucosa, sinuses, bronchii, and lungs.[4] This group includes:
- Flunisolide[5]
- Fluticasone propionate[5]
- Triamcinolone acetonide[5]
- Beclomethasone dipropionate[5]
- Budesonide[5]
There is also a combination preparation (trade name Advair), containing fluticasone propionate and salmeterol xinafoate (a long-acting bronchodilator).[5] It is approved for children over 12 years old.
Oral forms
Such as prednisone and prednisolone.[6]
Systemic forms
Available in injectables for intravenous and parenteral routes.[6]
Uses of corticosteroids
Synthetic pharmaceutical drugs with corticosteroid-like effect are used in a variety of conditions, ranging from brain tumors to skin diseases. Dexamethasone and its derivatives are almost pure glucocorticoids, while prednisone and its derivatives have some mineralocorticoid action in addition to the glucocorticoid effect. Fludrocortisone (Florinef) is a synthetic mineralocorticoid. Hydrocortisone (cortisol) is available for replacement therapy, e.g. in adrenal insufficiency and congenital adrenal hyperplasia.
Synthetic glucocorticoids are used in the treatment of joint pain or inflammation (arthritis), temporal arteritis, dermatitis, allergic reactions, asthma, hepatitis, systemic lupus erythematosus, inflammatory bowel disease (ulcerative colitis and Crohn's disease), sarcoidosis and for glucocorticoid replacement in Addison's disease or other forms of adrenal insufficiency. Topical formulations are also available for the skin, eyes (uveitis), lungs (asthma), nose (rhinitis), and bowels. Corticosteroids are also used supportively to prevent nausea, often in combination with 5-HT3 antagonists (e.g. ondansetron).
Typical undesired effects of glucocorticoids present quite uniformly as drug-induced Cushing's syndrome. Typical mineralocorticoid side-effects are hypertension (abnormally high blood pressure), hypokalemia (low potassium levels in the blood), hypernatremia (high sodium levels in the blood) without causing peripheral edema, metabolic alkalosis and connective tissue weakness.[7] There may also be impaired wound healing or ulcer formation because of the immunosuppressive effects.
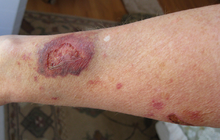
Clinical and experimental evidence indicates that corticosteroids can cause permanent eye damage by inducing central serous retinopathy (CSR, also known as central serous chorioretinopathy, CSC). A variety of steroid medications, from anti-allergy nasal sprays (Nasonex, Flonase) to topical skin creams, to eye drops (Tobradex), to prednisone have been implicated in the development of CSR.[8][9]
Corticosteroids have been widely used in treating people with traumatic brain injury.[citation needed] A systematic review identified 20 randomised controlled trials and included 12,303 participants, compared patients who received corticosteroids with patients who received no treatment. The authors recommended people with traumatic head injury should not be routinely treated with corticosteroids.[10]
History
First known use in 1944 according to: http://www.merriam-webster.com/dictionary/corticosteroid Tadeusz Reichstein together with Edward Calvin Kendall and Philip Showalter Hench were awarded the Nobel Prize for Physiology and Medicine in 1950 for their work on hormones of the adrenal cortex, which culminated in the isolation of cortisone.[11]
Corticosteroids have been used as drug treatment for some time. Lewis Sarett of Merck & Co. was the first to synthesize cortisone, using a complicated 36-step process that started with deoxycholic acid, which was extracted from ox bile.[12] The low efficiency of converting deoxycholic acid into cortisone led to a cost of US $200 per gram. Russell Marker, at Syntex, discovered a much cheaper and more convenient starting material, diosgenin from wild Mexican yams. His conversion of diosgenin into progesterone by a four-step process now known as Marker degradation was an important step in mass production of all steroidal hormones, including cortisone and chemicals used in hormonal contraception.[13] In 1952, D.H. Peterson and H.C. Murray of Upjohn developed a process that used Rhizopus mold to oxidize progesterone into a compound that was readily converted to cortisone.[14] The ability to cheaply synthesize large quantities of cortisone from the diosgenin in yams resulted in a rapid drop in price to US $6 per gram, falling to $0.46 per gram by 1980. Percy Julian's research also aided progress in the field.[15] The exact nature of cortisone's anti-inflammatory action remained a mystery for years after, however, until the leukocyte adhesion cascade and the role of phospholipase A2 in the production of prostaglandins and leukotrienes was fully understood in the early 1980s.
Side-effects
Long-term corticosteroids use has several severe side-effects as for example: hyperglycemia,[16] insulin resistance, diabetes mellitus,[16] osteoporosis, cataract, anxiety,[16] depression, colitis, hypertension, ictus, erectile dysfunction, hypogonadism, hypothyroidism, amenorrhoea, and retinopathy.
While the evidence for corticosteroids causing peptic ulceration is relatively poor except for high doses taken for over a month,[17] the majority of doctors as of 2010[update] still believe this is the case and would consider protective prophylaxis measures.[18]
Pregnancy
Corticosteroids have a small but significant teratogenic effect, causing a few birth defects per 1,000 pregnant women treated.[19]
Safety
Corticosteroids were voted Allergen of the Year in 2005 by the American Contact Dermatitis Society.[20]
See also
- Cushing's syndrome
- Addison's disease
- Diabetes mellitus
- Osteoporosis
- Cortisol
- Vitiligo
- Steroids (general term)
- Fluorometholone
- List of steroid abbreviations
- Topical steroid
References
- ^ Rietschel, Robert L. (2007). Fisher's Contact Dermatitis, 6/e. Hamilton, Ont: BC Decker Inc. pp. 256. ISBN 1-55009-378-9.
- ^ Coopman S, Degreef H, Dooms-Goossens A (July 1989). "Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids". Br. J. Dermatol. 121 (1): 27–34. doi:10.1111/j.1365-2133.1989.tb01396.x. PMID 2757954.
- ^ Wolverton, SE. Comprehensive Dermatologic Drug Therapy. WB Saunders, 2001. p. 562
- ^ http://www.webmd.com/asthma/guide/asthma_control_with_anti-inflammatory-drugs
- ^ a b c d e f New York City Asthma Initiative > INHALED CORTICOSTEROIDS - LONG-TERM CONTROL ASTHMA MEDICINE By Michael R. Bloomberg, Mayor, Thomas R. Frieden, M.D., M.P. H., Commissioner. 03/ 2004
- ^ a b http://dermnetnz.org/treatments/systemic-steroids.html
- ^ Werner R (2005). A massage therapist's guide to Pathology. 3rd edition. Lippincott Williams & Wilkins, Pennsylvania, USA.
- ^ Carvalho-Recchia, CA; Yannuzzi, LA; Negrão, S; Spaide, RF; Freund, KB; Rodriguez-Coleman, H; Lenharo, M; Iida, T (2002). "Corticosteroids and central serous chorioretinopathy". Ophthalmology 109 (10): 1834–7. doi:10.1016/S0161-6420(02)01117-X. PMID 12359603.
- ^ http://buteykola.com/2010/07/the-new-york-times-a-breathing-technique-offers-help-for-people-with-asthma
- ^ Alderson, P.; Roberts, I. (2005). "Corticosteroids for acute traumatic brain injury". In Alderson, Phil. Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD000196.pub2.
- ^ http://nobelprize.org/nobel_prizes/medicine/laureates/1950/kendall-lecture.pdf.
- ^ Sarett, Lewis H. (1947). “Process of Treating Pregnene Compounds”, U. S. Patent 2,462,133
- ^ Marker, Russell E.; Wagner, R. B.; Ulshafer, Paul R.; Wittbecker, Emerson L.; Goldsmith, Dale P. J.; Ruof, Clarence H. (1947). "Steroidal Sapogenins." J. Am. Chem. Soc. 69 (9): 2167. doi:10.1021/ja01201a032. PMID 20262743.
- ^ Peterson D.H., Murray, H.C. (1952). "Microbiological Oxygenation of Steroids at Carbon 11". J. Am. Chem. Soc., 74 (7): 1871–2. doi:10.1021/ja01127a531.
- ^ Julian, Percy L., Cole, John Wayne, Meyer, Edwin W., and Karpel, William J. (1956) “Preparation of Cortisone”. U. S. Patent 2,752,339
- ^ a b c Donihi AC, Raval D, Saul M, Korytkowski MT, DeVita MA (2006). "Prevalence and predictors of corticosteroid-related hyperglycemia in hospitalized patients". Endocr Pract 12 (4): 358–62. PMID 16901792.
- ^ Pecora PG, Kaplan B (1996). "Corticosteroids and ulcers: is there an association?". Ann Pharmacother 30 (7–8): 870–2. PMID 8826575.
- ^ Martínek J, Hlavova K, Zavada F, et al. (June 2010). ""A surviving myth" - corticosteroids are still considered ulcerogenic by a majority of physicians *". Scand J Gastroenterol 45 (10): 1156–61. doi:10.3109/00365521.2010.497935. PMID 20569095.
- ^ Shepard, TH.; Brent, RL.; Friedman, JM.; Jones, KL.; Miller, RK.; Moore, CA.; Polifka, JE. (Apr 2002). "Update on new developments in the study of human teratogens". Teratology 65 (4): 153–61. doi:10.1002/tera.10032. PMID 11948561.
- ^ http://www.medscape.com/viewarticle/505245
Cholesterol and steroid metabolic intermediates Mevalonate pathway to HMG-CoAto DMAPPGeranyl-Prephytoene diphosphate · PhytoeneNon-mevalonate pathway To Cholesterol Farnesyl pyrophosphate · Squalene · 2,3-Oxidosqualene · Lanosterol
Lanosterol · Lathosterol · 7-Dehydrocholesterol · Cholesterol
Lanosterol · Zymosterol · 7-Dehydrodesmosterol · Desmosterol · CholesterolSteroid Corticosteroids
(C21 pregnane)Nonhuman biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iPharmacology: major drug groups Gastrointestinal tract/metabolism (A) stomach acid (Antacids, H2 antagonists, Proton pump inhibitors) • Antiemetics • Laxatives • Antidiarrhoeals/Antipropulsives • Anti-obesity drugs • Anti-diabetics • Vitamins • Dietary mineralsBlood and blood forming organs (B) Cardiovascular system (C) cardiac therapy/antianginals (Cardiac glycosides, Antiarrhythmics, Cardiac stimulants)
Antihypertensives • Diuretics • Vasodilators • Beta blockers • Calcium channel blockers • renin-angiotensin system (ACE inhibitors, Angiotensin II receptor antagonists, Renin inhibitors)
Antihyperlipidemics (Statins, Fibrates, Bile acid sequestrants)Skin (D) Genitourinary system (G) Endocrine system (H) Hypothalamic-pituitary hormones • Corticosteroids (Glucocorticoids, Mineralocorticoids) • Sex hormones • Thyroid hormones/Antithyroid agentsInfections and infestations (J, P, QI) Antimicrobials: Antibacterials (Antimycobacterials) • Antifungals • Antivirals • Antiparasitics (Antiprotozoals, Anthelmintics, Ectoparasiticides) • IVIG • VaccinesMalignant disease (L01-L02) Immune disease (L03-L04) Muscles, bones, and joints (M) Anabolic steroids • Anti-inflammatories (NSAIDs) • Antirheumatics • Corticosteroids • Muscle relaxants • BisphosphonatesBrain and nervous system (N) Analgesics • Anesthetics (General, Local) • Anorectics • Anti-ADHD Agents • Antiaddictives • Anticonvulsants • Antidementia Agents • Antidepressants • Antimigraine Agents • Antiparkinson's Agents • Antipsychotics • Anxiolytics • Depressants • Entactogens • Entheogens • Euphoriants • Hallucinogens (Psychedelics, Dissociatives, Deliriants) • Hypnotics/Sedatives • Mood Stabilizers • Neuroprotectives • Nootropics • Neurotoxins • Orexigenics • Serenics • Stimulants • Wakefulness-Promoting AgentsRespiratory system (R) Sensory organs (S) Other ATC (V) Categories:- Corticosteroids
Wikimedia Foundation. 2010.