- Metabolic alkalosis
-
Metabolic alkalosis Classification and external resources 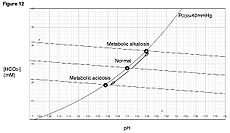
Davenport diagramICD-10 E87.3 ICD-9 276.3 DiseasesDB 402 eMedicine med/1459 Metabolic alkalosis is a metabolic condition in which the pH of tissue is elevated beyond the normal range ( 7.35-7.45 ). This is the result of decreased hydrogen ion concentration, leading to increased bicarbonate, or alternatively a direct result of increased bicarbonate concentrations.
Contents
Terminology
- Alkalosis refers to a high pH in tissue.
- Alkalemia refers to a high pH in the blood.
Causes
There are five main causes of metabolic alkalosis.[1]
These can be divided into two categories, depending upon urine chloride levels.[2]
Chloride-responsive (<10 mEq/L)
- Loss of hydrogen ions - Most often occurs via two mechanisms, either vomiting or via the kidney.
- Vomiting results in the loss of hydrochloric acid (hydrogen and chloride ions) with the stomach contents. In the hospital setting this can commonly occur from nasogastric suction tubes.
- Severe vomitting also causes loss of potassium (hypokalaemia) and sodium (hyponatraemia). The kidneys compensate for these losses by retaining sodium in the collecting ducts at the expense of hydrogen ions (sparing sodium/potassium pumps to prevent further loss of potassium), leading to metabolic alkalosis.
- Congenital chloride diarrhea - rare for being a diarrhea that causes alkalosis instead of acidosis.
- Contraction alkalosis - This results from a loss of water in the extracellular space which is poor in bicarbonate, typically from diuretic use. Since water is lost while bicarbonate is retained, the increased concentration of bicarbonate "mops up" more of the hydrogen ions and raises the blood pH.
- Diuretic therapy - loop diuretics and thiazides can both initially cause increase in chloride, but once stores are depleted, urine excretion will be below < 25 mEq/L. The loss of fluid from sodium excretion causes a contraction alkalosis.
- Posthypercapnia - Hypercapnia causes respiratory acidosis. Renal compensation with excess bicarbonate occurs to lessen the affect of the acidosis. Once carbon dioxide levels return to base line, the higher bicarbonate levels reveal themselves putting the patient into metabolic alkalosis
Chloride-resistant (>20 mEq/L)
- Retention of bicarbonate
- Shift of hydrogen ions into intracellular space - Seen in hypokalemia. Due to a low extracellular potassium concentration, potassium shifts out of the cells. In order to maintain electrical neutrality, hydrogen shifts into the cells, raising blood pH.
- Alkalotic agents - Alkalotic agents, such as bicarbonate (administrated in cases of peptic ulcer or hyperacidity) or antacids, administered in excess can lead to an alkalosis.
- Hyperaldosteronism - Renal loss of hydrogen ions occurs when excess aldosterone (Conn's syndrome) increases the activity of a sodium-hydrogen exchange protein in the kidney. This increases the retention of sodium ions whilst pumping hydrogen ions into the renal tubule. Excess sodium increases extracellular volume and the loss of hydrogen ions creates a metabolic alkalosis. Later, the kidney responds through the aldosterone escape to excrete sodium and chloride in urine.[3]
- Licorice
- Bartter syndrome - a syndrome analogous to taking loop diuretics characterized with normotensive patients
- Liddle syndrome - a syndrome from defect sodium channel deletion characterized by hypertension and hypoaldosteronism.
- 11β-hydroxylase deficiency and 17 alpha-hydroxylase deficiency - both characterized by hypertension
Compensation
Compensation for metabolic alkalosis occurs mainly in the lungs, which retain carbon dioxide (CO2) through slower breathing, or hypoventilation (respiratory compensation). CO2 is then consumed toward the formation of the carbonic acid intermediate, thus decreasing pH. Respiratory compensation, though, is incomplete. The decrease in [H+] suppresses the peripheral chemoreceptors, which are sensitive to pH. But, because respiration slows, there's an increase in PCO2 which would cause an offset of the depression because of the action of the central chemoreceptors which are sensitive to the partial pressure of CO2 in the blood. So, because of the central chemoreceptors, respiration rate would be increased.
Renal compensation for metabolic alkalosis, less effective than respiratory compensation, consists of increased excretion of HCO3- (bicarbonate), as the filtered load of HCO3- exceeds the ability of the renal tubule to reabsorb it.
See also
References
- ^ eMedicine - Metabolic Alkalosis : Article by Sameer Yaseen
- ^ "Alkalosis, Metabolic: eMedicine Pediatrics: Cardiac Disease and Critical Care Medicine". http://emedicine.medscape.com/article/906819-overview. Retrieved 2009-05-10.
- ^ Cho Kerry C, "Chapter 21. Electrolyte & Acid-Base Disorders" (Chapter). McPhee SJ, Papadakis MA: CURRENT Medical Diagnosis & Treatment 2011: http://www.accessmedicine.com/content.aspx?aID=10909.
Water-electrolyte imbalance and acid-base imbalance (E86–E87, 276) Volume status Electrolyte Acid-base Metabolic: High anion gap (Ketoacidosis/Diabetic ketoacidosis, Lactic) · Normal anion gap (Hyperchloremic, Renal tubular)Metabolic: Contraction alkalosisBothCategories:- Acid-base disturbances
Wikimedia Foundation. 2010.
