- Respiratory acidosis
Infobox_Disease
Name = PAGENAME
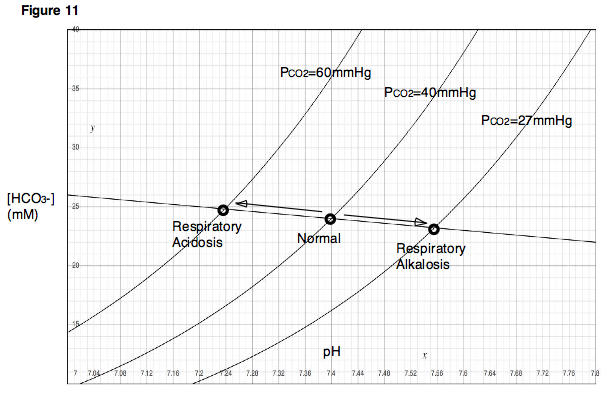
Caption =Davenport diagram
DiseasesDB = 95
ICD10 = ICD10|E|87|2|e|70
ICD9 = ICD9|276.2
ICDO =
OMIM =
MedlinePlus =
eMedicineSubj = med
eMedicineTopic = 2008
MeshID = D000142Respiratory acidosis is
acidosis (abnormally increased acidity of the blood) due to decreased ventilation of the pulmonaryalveoli , leading to elevated arterialcarbon dioxide concentration ("Pa"CO2).Respiratory acidosis is a clinical disturbance that is due to alveolar hypoventilation. Production of carbon dioxide occurs rapidly, and failure of ventilation promptly increases the level of "Pa"CO2. Alveolar hypoventilation leads to an increased "Pa"CO2 (ie,
hypercapnia ). The increase in "Pa"CO2 in turn decreases the HCO3-/"Pa"CO2 ratio and decreasespH .Hypercapnia and respiratory acidosis occur when impairment in ventilation occurs and the removal of CO2 by the lungs is less than the production of CO2 in the tissues.Types of respiratory acidosis
Respiratory acidosis can be acute or chronic.
* In "acute respiratory acidosis", the "Pa"CO2 is elevated above the upper limit of the reference range (over 6.3 kPa or 47 mm Hg) with an accompanying acidemia (pH <7.35).
* In "chronic respiratory acidosis", the "Pa"CO2 is elevated above the upper limit of the reference range, with a normal blood pH (7.35 to 7.45) or near-normal pH secondary torenal compensation and an elevated serum bicarbonate (HCO3- >30 mm Hg).Causes
Acute
Acute respiratory acidosis occurs when an abrupt failure of ventilation occurs. This failure in ventilation may be caused by depression of the
central respiratory center by cerebral disease or drugs, inability to ventilate adequately due toneuromuscular disease (eg,myasthenia gravis ,amyotrophic lateral sclerosis ,Guillain-Barré syndrome ,muscular dystrophy ), or airway obstruction related to asthma or chronic obstructive pulmonary disease (COPD) exacerbation.Chronic
Chronic respiratory acidosis may be secondary to many disorders, including
COPD . Hypoventilation in COPD involves multiple mechanisms, including decreased responsiveness to hypoxia andhypercapnia , increasedventilation-perfusion mismatch leading to increaseddead space ventilation, and decreased diaphragm function secondary to fatigue and hyperinflation.Chronic respiratory acidosis also may be secondary to
obesity hypoventilation syndrome (ie,Pickwickian syndrome ), neuromuscular disorders such asamyotrophic lateral sclerosis , and severe restrictive ventilatory defects as observed ininterstitial fibrosis andthoracic deformities.Lung diseases that primarily cause abnormality in
alveolar gas exchange usually do not cause hypoventilation but tend to cause stimulation of ventilation and hypocapnia secondary to hypoxia. Hypercapnia only occurs if severe disease or respiratory muscle fatigue occurs.Physiological response
Mechanism
Metabolism rapidly generates a large quantity of volatile acid (H2CO3) and
nonvolatile acid . The metabolism of fats and carbohydrates leads to the formation of a large amount of CO2. The CO2 combines with H2O to formcarbonic acid (H2CO3). The lungs excrete the volatile fraction through ventilation, and acid accumulation does not occur. A significant alteration in ventilation that affects elimination of CO2 can cause a respiratory acid-base disorder. The "Pa"CO2 is maintained within a range of 39-41 mm Hg in normal states.Alveolar ventilation is under the control of the central respiratory centers, which are located in the
pons and themedulla . Ventilation is influenced and regulated bychemoreceptors for "Pa"CO2, PaO2, and pH located in the brainstem,and in theaortic and carotid bodies as well as by neural impulses from lungstretch receptors and impulses from thecerebral cortex . Failure of ventilation quickly increases the "Pa"CO2.In acute respiratory acidosis, compensation occurs in 2 steps.
* The initial response is cellular buffering that occurs over minutes to hours. Cellular buffering elevates plasma bicarbonate (HCO3-) only slightly, approximately 1 mEq/L for each 10-mm Hg increase in "Pa"CO2.
* The second step is renal compensation that occurs over 3-5 days. With renal compensation, renal excretion of carbonic acid is increased and bicarbonate reabsorption is increased. For instance,PEPCK is upregulated inrenal proximal tubule brush border cell s, in order to secrete more NH3 and thus to produce more HCO3-. cite book |author=Walter F., PhD. Boron |title=Medical Physiology: A Cellular And Molecular Approaoch |publisher=Elsevier/Saunders |location= |year= |pages= |isbn=1-4160-2328-3 |oclc= |doi= Page 858 ]Estimated changes
In renal compensation, plasma bicarbonate rises 3.5 mEq/L for each increase of 10 mm Hg in "Pa"CO2. The expected change in serum bicarbonate concentration in respiratory acidosis can be estimated as follows:
* Acute respiratory acidosis: HCO3- increases 1 mEq/L for each 10-mm Hg rise in "Pa"CO2.
* Chronic respiratory acidosis: HCO3- rises 3.5 mEq/L for each 10-mm Hg rise in "Pa"CO2.
The expected change in pH with respiratory acidosis can be estimated with the following equations:
* Acute respiratory acidosis: Change in pH = 0.008 X (40 - "Pa"CO2)
* Chronic respiratory acidosis: Change in pH = 0.003 X (40 - "Pa"CO2)
Respiratory acidosis does not have a great effect on
electrolyte levels. Some small effects occur on calcium and potassium levels. Acidosis decreases binding of calcium to albumin and tends to increase serum ionized calcium levels. In addition, acidemia causes an extracellular shift of potassium, but respiratory acidosis rarely causes clinically significanthyperkalemia .References
External links
*
Wikimedia Foundation. 2010.
