- Difluprednate
-
Difluprednate 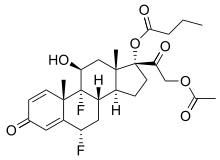
Systematic (IUPAC) name [(6S,8S,9R,10S,11S,13S,14S,17R)-17-(2-acetyloxyacetyl)-6,9-difluoro-11-hydroxy-10,13-dimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] butanoate Clinical data AHFS/Drugs.com monograph MedlinePlus a609025 Licence data US FDA:link Pregnancy cat. ? Legal status ℞-only (US) Routes topical dermatologic Identifiers CAS number 23674-86-4 
ATC code D07AC19 PubChem CID 32037 DrugBank DB06781 ChemSpider 391990 
UNII S8A06QG2QE 
KEGG D01266 
ChEMBL CHEMBL1201749 
Chemical data Formula C27H34F2O7 Mol. mass 508.551  (what is this?) (verify)
(what is this?) (verify)Difluprednate is a corticosteroid, It is chemically a butyrate ester of 6(alpha),9(alpha)-difluoro prednisolone acetate. Accordingly, difluprednate is sometimes abbreviated DFBA, for difluoroprednisolone butyrate acetate.
Approval
On June 24, 2008, the US Food and Drug Administration (FDA) approved difluprednate for the treatment of post-operative ocular inflammation and pain.[1] It is marketed by Alcon under the tradename Durezol.
Clinical trials
Difluprednate ophthalmic emulsion 0.05% is also being studied in other ocular inflammatory diseases, including a U.S. Phase 3 study evaluating difluprednate for the treatment of anterior uveitis.[2]
References
- ^ "Sirion Therapeutics Announces FDA Approval of Durezol for Treatment of Postoperative Ocular Inflammation and Pain" (Press release). Sirion Therapeutics, Inc.. 2008-06-24. http://www.drugs.com/newdrugs/sirion-therapeutics-announces-fda-approval-durezol-postoperative-ocular-inflammation-pain-1031.html. Retrieved 2008-06-30.
- ^ ClinicalTrials.gov
Categories:- Organofluorides
- Butyrates
- Acetate esters
- Corticosteroids
Wikimedia Foundation. 2010.
