- Amiodarone
-
Amiodarone 
Systematic (IUPAC) name (2-{4-[(2-butyl-1-benzofuran-3-yl)carbonyl]-2,6-diiodophenoxy}ethyl)diethylamine Clinical data Trade names Cordarone AHFS/Drugs.com monograph MedlinePlus a687009 Pregnancy cat. D(US) Legal status ℞ Prescription only Routes oral or intravenous Pharmacokinetic data Bioavailability 20 - 55% Metabolism Liver Half-life 58 days (range 15-142 days) Excretion Primarily Hepatic and Biliary Identifiers CAS number 1951-25-3 
ATC code C01BD01 PubChem CID 2157 DrugBank APRD00288 ChemSpider 2072 
UNII N3RQ532IUT 
KEGG D02910 
ChEBI CHEBI:2663 
ChEMBL CHEMBL633 
Chemical data Formula C25H29I2NO3 Mol. mass 645,31 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Amiodarone is an antiarrhythmic agent (medication used for irregular heart beat) used for various types of tachyarrhythmias (fast forms of irregular heart beat), both ventricular and supraventricular (atrial) arrhythmias. Discovered in 1961, it was not approved for use in the United States until 1985. Despite relatively common side-effects, it is used in arrhythmias that are otherwise difficult to treat with medication. Related newer compounds, such as dronedarone, have lower efficacy but a reduced rate of side-effects.
Contents
History
Amiodarone was initially developed in 1961 at the Labaz company, Belgium, by chemists Tondeur and Binon. It became popular in Europe as a treatment for angina pectoris.[1][2]
As a doctoral candidate at Oxford University, Dr. Bramah Singh determined that amiodarone and sotalol had antiarrhythmic properties and belonged to a new class of antiarrhythmic agents (what would become the class III antiarrhythmic agents).[3] Today the mechanisms of action of amiodarone and sotalol remain unknown. It is thought to involve prolonging the action potential duration, prolonging the refractory period, or interacting with K+ channels.
Based on Singh's work, the Argentinian physician Dr. Mauricio Rosenbaum began using amiodarone to treat his patients who suffered from supraventricular and ventricular arrhythmias, with impressive results. Based on papers written by Dr. Rosenbaum developing Singh's theories, physicians in the United States began prescribing amiodarone to their patients with potentially life-threatening arrhythmias in the late 1970s.[4][5] By 1980, amiodarone was commonly prescribed throughout Europe for the treatment of arrhythmias, but in the U.S. amiodarone remained unapproved by the Food and Drug Administration, and physicians were forced to directly obtain amiodarone from pharmaceutical companies in Canada and Europe.[citation needed]
The FDA was reluctant to officially approve the use of amiodarone, since initial reports had shown increased incidence of serious pulmonary side-effects of the drug. In the mid 1980s, the European pharmaceutical companies began putting pressure on the FDA to approve amiodarone by threatening to cut the supply to American physicians if it were not approved. In December 1985, amiodarone was approved by the FDA for the treatment of arrhythmias.[6] This makes amiodarone one of the few drugs approved by the FDA without rigorous randomized clinical trials.
Dosing
Amiodarone is available in oral and intravenous formulations.
Orally, it is available under the trade names Pacerone (produced by Upsher-Smith Laboratories, Inc.) and Cordarone (produced by Wyeth-Ayerst Laboratories) in 200 mg and 400 mg tablets; It is also available under the trade name Aratac (produced by Alphapharm Pty Ltd) in 100 mg and 200 mg tablets in Australia and New Zealand. Also Arycor in South Africa (Produced by Winthrop Pharmaceuticals.) in doses of 100 mg and 200 mg scored tablets. In South America, it is known as Atlansil and is produced by Roemmers.
It is also available in intravenous ampules and vials, typically in 150 mg increments.
The dose of amiodarone administered is tailored to the individual and the dysrhythmia that is being treated. When administered orally, the bioavailability of amiodarone is quite variable. Absorption ranges from 22 to 95%, with better absorption when it is given with food.[7]
Amiodarone is fat-soluble, and tends to concentrate in tissues including fat, muscle, liver, lungs, and skin. This confers a high volume of distribution (5000 liters in a 70 kg adult) and a long half-life. Due to the long half-life of amiodarone, oral loading typically takes days to weeks.
An oral loading dose is typically a total of 10 grams, divided over one to two weeks but there are many other dosing regimens. Once an individual is loaded, a typical maintenance dose of amiodarone is 100 or 200 mg either once or twice daily.
An intravenous loading dose is typically 300 mg in 20-30cc D5W for cardiac arrest. The loading infusion for dysrhythmias is typically 150 mg in a 100cc bag of D5W given over 10 minutes. Both can be followed by a 360 mg slow infusion over 6 hours then a maintenance infusion of 540 mg over 18 hours.
Mechanism of action
Amiodarone is categorized as a class III antiarrhythmic agent, and prolongs phase 3 of the cardiac action potential. It has numerous other effects however, including actions that are similar to those of antiarrhythmic classes Ia, II, and IV.
Amiodarone shows beta blocker-like and potassium channel blocker-like actions on the SA and AV nodes, increases the refractory period via sodium- and potassium-channel effects, and slows intra-cardiac conduction of the cardiac action potential, via sodium-channel effects.
Amiodarone resembles thyroid hormone, and its binding to the nuclear thyroid receptor might contribute to some of its pharmacologic and toxic actions [8]
Indications for use
Because amiodarone has a low incidence of pro-arrhythmic effects, it has been used both in the treatment of acute life-threatening arrhythmias as well as the chronic suppression of arrhythmias. It is useful both in supraventricular arrhythmias and ventricular arrhythmias.
Ventricular fibrillation
The treatment of choice for ventricular fibrillation (VF) is electrical defibrillation. However, amiodarone can be useful in shock-refractory VF. In the ARREST trial, amiodarone was shown to improve survival to hospital admission (when compared to placebo) in individuals who suffer cardiac arrest with shock-refractory VF.[9] It is on the basis of this study that the guidelines created by the American Heart Association for the treatment of VF include amiodarone as a second line agent (after epinephrine or vasopressin). ARREST was not adequately powered to demonstrate survival to hospital discharge.
Ventricular tachycardia
Amiodarone may be used in the treatment of ventricular tachycardia in certain instances. Individuals with hemodynamically unstable ventricular tachycardia should not initially receive amiodarone. These individuals should be cardioverted out of their unstable rhythm.
Amiodarone can be used in individuals with hemodynamically stable ventricular tachycardia. In these cases, amiodarone can be used regardless of the individual's underlying heart function and the type of ventricular tachycardia; it can be used in individuals with monomorphic ventricular tachycardia, but is contraindicated in individuals with polymorphic ventricular tachycardia as it is associated with a prolong QT interval which will be made worse with anti-arrhythmic drugs. The dose of amiodarone is 150 mg IV administered over 10 minutes.
Atrial fibrillation
Individuals who have undergone open heart surgery are at an increased risk of developing atrial fibrillation (or AF) in the first few days post-procedure. In the ARCH trial, intravenous amiodarone (2 grams administered over 2 days) has been shown to reduce the incidence of atrial fibrillation after open heart surgery when compared to placebo.[10] However, clinical studies have failed to demonstrate long-term efficacy and have shown potentially fatal side effects such as pulmonary toxicities. While Amiodarone is not approved for AF by the FDA, it is a commonly prescribed off-label treatment due to the lack of efficacious treatment alternatives.
So called 'acute onset atrial fibrillation', defined by the North American Society of Pacing and Electrophysiology (NASPE) in 2003, responds well to short duration treatment with amiodarone. This has been demonstrated in seventeen randomised controlled trials, of which five included a placebo arm. The incidence of severe side effects in this group is low.
The benefit of amiodarone in the treatment of atrial fibrillation in the critical care population has yet to be determined but it may prove to be the agent of choice where the patient is haemodynamically unstable and unsuitable for DC cardioversion. It is recommended in such a role by the UK government's National Institute for Health and Clinical Excellence (NICE).
Contraindications
Individuals who are pregnant or may become pregnant are strongly advised to not take amiodarone. Since amiodarone can be expressed in breast milk, women taking amiodarone are advised to stop nursing.
It is contraindicated in individuals with sinus nodal bradycardia, atrioventricular block, and second or third degree heart block who do not have an artificial pacemaker.
Individuals with baseline depressed lung function should be monitored closely if amiodarone therapy is to be initiated.
The injection should not be given to neonates, because the benzyl alcohol it contains may cause the fatal "gasping syndrome".
Amiodarone can worsen the cardiac arrhythmia brought on by Digitalis toxicity.
Not to be given with Lidocaine → increases risk of asystole[citation needed]
Metabolism
Amiodarone is extensively metabolized in the liver by cytochrome P450 3A4, and can affect the metabolism of numerous other drugs. It interacts with digoxin, warfarin, phenytoin and others. The major metabolite of amiodarone is desethylamiodarone (DEA), which also has antiarrhythmic properties. The metabolism of amiodarone is inhibited by grapefruit juice, leading to elevated serum levels of amiodarone.
On August 8, 2008 FDA issued a warning of the risk of rhabdomyolysis, which can lead to kidney failure or death, when simvastatin is used with amiodarone. This interaction is dose-dependent with simvastatin doses exceeding 20 mg. This drug combination especially with higher doses of simvastatin should be avoided.[11]
Interactions with other drugs
The pharmacokinetics of numerous drugs, including many that are commonly administered to individuals with heart disease, are affected by amiodarone. Particularly, doses of digoxin should be halved in individuals taking amiodarone.
Amiodarone potentiates the action of warfarin. Individuals taking both of these medications should have their warfarin dose halved and their anticoagulation status (measured as prothrombin time (PT) and international normalized ratio (INR)) measured more frequently. The effect of amiodarone in the warfarin concentration can be as early as a few days after initiation of treatment, or can be delayed a few weeks.
Amiodarone inhibits the action of the cytochrome P450 isozyme family. This reduces the clearance of many drugs, including the following: -
- Cyclosporine
- Digoxin
- Flecainide
- Procainamide
- Quinidine
- Sildenafil
- Simvastatin
- Theophylline
- Warfarin
Excretion
Excretion is primarily hepatic and biliary with almost no elimination via the renal route and it is not dialyzable [Package Insert- Pacerone(R)]. Elimination half-life average of 58 days (ranging from 25–100 days [Remington: The Science and Practice of Pharmacy 21st edition]) for amiodarone and 36 days for the active metabolite, desethylamiodarone (DEA) [Package Insert- Pacerone(R)]. There is 10-50% transfer of amiodarone and DEA in the placenta as well as presence in breast milk [Package Insert- Pacerone(R)]. Accumulation of amiodarone and DEA occurs in adipose tissue and highly perfused organs (i.e. liver, lungs) [Package Insert- Pacerone(R)], therefore, if an individual was taking amiodarone on a chronic basis, if it is stopped it will remain in the system for weeks to months.
Side effects
Amiodarone has numerous side effects. Most individuals administered amiodarone on a chronic basis will experience at least one side effect.
Lung
The most serious reaction that is due to amiodarone is interstitial lung disease. Risk factors include high cumulative dose, more than 400 milligrams per day, duration over two months, increased age, and preexisting pulmonary disease. Some individuals were noted to develop pulmonary fibrosis after a week of treatment, while others did not develop it after years of continuous use. Common practice is to avoid the agent if possible in individuals with decreased lung function.
The most specific test of pulmonary toxicity due to amiodarone is a dramatically decreased DLCO noted on pulmonary function testing.
Thyroid
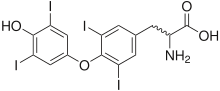 Thyroxine and amiodarone have similar structures.
Thyroxine and amiodarone have similar structures.
Due to the iodine content of the agent (37.3% by weight), abnormalities in thyroid function are common. Amiodarone is structurally similar to thyroxine (a thyroid hormone), which contributes to the effects of amiodarone on thyroid function.[citation needed] Both under- and overactivity of the thyroid may occur on amiodarone treatment. Measurement of free thyroxine (FT4) alone may be unreliable in detecting these problems and thyroid-stimulating hormone (TSH) should therefore also be checked every 6 months.[12]
- Hypothyroidism (slowing of the thyroid, due to the Wolff-Chaikoff effect) occurs frequently; in the SAFE trial, which compared amiodarone with other medications for the treatment of atrial fibrillation, biochemical hypothyroidism (as defined by a TSH level of 4.5-10 mU/l) occurred in 25.8% of the amiodarone-treated group as opposed to 6.6% of the control group (taking placebo or sotalol). Overt hypothyroidism (defined as TSH >10 mU/l) occurred at 5.0% compared to 0.3%; most of these (>90%) were detected within the first six months of amiodarone treatment.[13]
- Hyperthyroidism (an overactive thyroid, due to the Jod-Basedow Effect) can also occur. However, in the SAFE trial, the increased rate of hyperthyroidism (5.3% compared to 2.4%) was not statistically significant. Most hyperthyroid patients (defined as TSH <0.35 mU/l) were asymptomatic.[13]
Thyroid uptake measurements (I-123 or I-131), which are used to differentiate causes of hyperthyroidism, are generally unreliable in patients who have been taking amiodarone. Because of the high iodine content of amiodarone, the thyroid gland is effectively saturated, thus preventing further uptake of isotopes of iodine. However, the radioactive iodine uptake (nuclear thyroid uptake test) may still be helpful in the diagnosis and management of amiodarone-induced hyperthyroidism.[citation needed]
Eye
Corneal micro-deposits (Corneal verticillata, also called vortex keratopathy) are almost universally present (over 90%) in individuals taking amiodarone for at least 6 months. These deposits typically do not cause any symptoms. About 1 in 10 individuals may complain of a bluish halo. Optic neuropathy occurs in 1-2% of people and is not dosage dependent. Bilateral optic disk swelling and mild and reversible visual field defects can also occur.
Gastrointestinal system and liver
Abnormal liver enzyme results are common in patients on amiodarone. Much rarer are jaundice, hepatomegaly (liver enlargement), and hepatitis (inflammation of the liver).[14]
Low-dose amiodarone has been reported to cause pseudo-alcoholic cirrhosis.[15][16]
Skin
Long-term administration of amiodarone is associated with a blue-grey discoloration of the skin. This is more commonly seen in individuals with lighter skin tones. The discoloration may revert upon cessation of the drug. However, the skin color may not return completely to normal.
Individuals taking amiodarone may become more sensitive to the harmful effects of UV-A light. Using sunblock that also blocks UV-A rays appears to prevent this side effect.
Neurological
Long-term administration of amiodarone has been associated with peripheral neuropathies.[17]
Epididymis
Amiodarone is sometimes responsible for epididymitis, a condition of the scrotum normally associated with bacterial infections but which can also occur as a non-bacterial inflammatory condition. Amiodarone accumulates in the head of the organ and can cause unilateral or bilateral inflammation. It tends to resolve if amiodarone is stopped.[18]
Gynecomastia
Some cases of gynecomastia have been reported with men on amiodarone.[19]
See also
- Advanced cardiac life support (ACLS)
- Antiarrhythmic agents
- Atrial fibrillation
- Cardiac action potential
- Dronedarone (similar drug under investigation)
- Ventricular tachycardia
References
- ^ Deltour G, Binon F, Tondeur R, et al. (1962). "[Studies in the benzofuran series. VI. Coronary-dilating activity of alkylated and aminoalkylated derivatives of 3-benzoylbenzofuran.]" (in French). Archives internationales de pharmacodynamie et de thérapie 139: 247–54. PMID 14026835.
- ^ Charlier R, Deltour G, Tondeur R, Binon F (1962). "[Studies in the benzofuran series. VII. Preliminary pharmacological study of 2-butyl-3-(3,5-diiodo-4-beta-N-diethylaminoethoxybenzoyl)-benzofuran.]" (in French). Archives internationales de pharmacodynamie et de thérapie 139: 255–64. PMID 14020244.
- ^ Singh BN, Vaughan Williams EM (1970). "The effect of amiodarone, a new anti-anginal drug, on cardiac muscle". Br. J. Pharmacol. 39 (4): 657–67. PMC 1702721. PMID 5485142. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1702721.
- ^ Rosenbaum MB, Chiale PA, Halpern MS, et al. (1976). "Clinical efficacy of amiodarone as an antiarrhythmic agent". Am. J. Cardiol. 38 (7): 934–44. doi:10.1016/0002-9149(76)90807-9. PMID 793369.
- ^ Rosenbaum MB, Chiale PA, Haedo A, Lázzari JO, Elizari MV (1983). "Ten years of experience with amiodarone". Am. Heart J. 106 (4 Pt 2): 957–64. doi:10.1016/0002-8703(83)90022-4. PMID 6613843.
- ^ "Drug Approval Package". http://www.fda.gov/cder/foi/nda/pre96/18-972_Cardarone.htm. Retrieved 2007-09-30.[dead link]
- ^ Siddoway LA (2003). "Amiodarone: guidelines for use and monitoring". American Family Physician 68 (11): 2189–96. PMID 14677664. http://www.aafp.org/afp/20031201/2189.html.
- ^ Goodman & Gilman's The Pharmacological Basis of Therapeutics, 11th Edition.
- ^ Kudenchuk PJ, Cobb LA, Copass MK, et al. (1999). "Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation". N. Engl. J. Med. 341 (12): 871–8. doi:10.1056/NEJM199909163411203. PMID 10486418.
- ^ Guarnieri T, Nolan S, Gottlieb SO, Dudek A, Lowry DR (1999). "Intravenous amiodarone for the prevention of atrial fibrillation after open heart surgery: the Amiodarone Reduction in Coronary Heart (ARCH) trial". J. Am. Coll. Cardiol. 34 (2): 343–7. doi:10.1016/S0735-1097(99)00212-0. PMID 10440143.
- ^ "Information on Simvastatin/Amiodarone". http://www.fda.gov/cder/drug/infopage/simvastatin_amiodarone/default.htm. Retrieved 2008-09-21.[dead link]
- ^ British National Formulary guidance on thyroid function monitoring (BNF Amiodarone)
- ^ a b Batcher EL, Tang XC, Singh BN, Singh SN, Reda DJ, Hershman JM (October 2007). "Thyroid function abnormalities during amiodarone therapy for persistent atrial fibrillation". Am J Med 120 (10): 880–85. doi:10.1016/j.amjmed.2007.04.022. PMID 17904459. http://www.amjmed.com/article/PIIS0002934307004718/abstract.
- ^ Flaharty KK, Chase SL, Yaghsezian HM, Rubin R (1989). "Hepatotoxicity associated with amiodarone therapy". Pharmacotherapy 9 (1): 39–44. PMID 2646621.
- ^ Singhal A, Ghosh P, Khan SA (2003). "Low dose amiodarone causing pseudo-alcoholic cirrhosis". Age and ageing 32 (2): 224–5. doi:10.1093/ageing/32.2.224. PMID 12615569.
- ^ Puli SR, Fraley MA, Puli V, Kuperman AB, Alpert MA (2005). "Hepatic cirrhosis caused by low-dose oral amiodarone therapy". Am. J. Med. Sci. 330 (5): 257–61. doi:10.1097/00000441-200511000-00012. PMID 16284489.
- ^ A G Fraser, I N McQueen, A H Watt, and M R Stephens (June 1985). "Peripheral neuropathy during longterm high-dose amiodarone therapy". J Neurol Neurosurg Psychiatry 48 (6): 576–578. doi:10.1136/jnnp.48.6.576. PMC 1028375. PMID 2989436. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1028375/.
- ^ Thomas A, Woodard C, Rovner ES, Wein AJ (February 2003). "Urologic complications of nonurologic medications". Urol. Clin. North Am. 30 (1): 123–31. doi:10.1016/S0094-0143(02)00111-8. PMID 12580564.
- ^ [1] Gynecomastia: Its features, and when and how to treat it
External links
- Siddoway LA (December 2003). "Amiodarone: guidelines for use and monitoring". Am Fam Physician 68 (11): 2189–96. PMID 14677664. http://www.aafp.org/afp/20031201/2189.html.
- Amiodarone (MedicineNet.com)
- Amiodarone (FamilyPracticeNotebook.com)
- Amiodarone (The WorldWide Intensivist)
- U.S. National Library of Medicine: Drug Information Portal - Amiodarone
- Amiodarone (FDA MedWatch)
Channel blocker: potassium channel blockers Antiarrhythmic III/delayed rectifier benzofuran (Amiodarone) • quaternary ammonium (Bretylium) • naphthalene (Bunaftine) • phenethylamine (Dofetilide) • sulfonamide (Ibutilide) • pyrimidinone (Nifekalant) • ethanolamine (Sotalol) • cyclopropane (Tedisamil) • E-4031Other/ungrouped/unknown aminopyridines (3,4-Diaminopyridine, 4-Aminopyridine) • indole (Linopirdine, Paxilline) • quaternary ammonium (Tetraethylammonium) • peptide (Maurotoxin, Charybdotoxin)Antiarrhythmic agents (C01B) Channel blockers class Ib (Phase 3←)class Ic (Phase 0→)Amiodarone • Dronedarone • Bretylium • Bunaftine • Dofetilide • Ibutilide • Nifekalant • Sotalol • Tedisamil • Vernakalant • E-4031Receptor agonists
and antagonistsIon transporters Health science > Medicine > Emergency medicine Procedures Acute Care of at-Risk Newborns (ACoRN) · Advanced cardiac life support (ACLS) · Advanced Trauma Life Support (ATLS) · Cardiopulmonary resuscitation (CPR) · First aid · Neonatal Resuscitation Program (NRP) · Pediatric Advanced Life Support (PALS) · Basic Life SupportEquipment Bag valve mask (BVM) · Chest tube · Defibrillation (AED, ICD) · Electrocardiogram (ECG/EKG) · Intraosseous infusion (IO) · Intravenous therapy (IV) · Tracheal intubation · Nasopharyngeal airway (NPA) · Oropharyngeal airway (OPA) · Pocket maskDrugs Other Categories:- Amines
- Antiarrhythmic agents
- Benzofurans
- Organoiodides
- Aromatic ketones
- Phenol ethers
- Potassium channel blockers
Wikimedia Foundation. 2010.