- Benzofuran
-
This article is about the heterocyclic chemical compound. For its purportedly recreational derivative drug, nicknamed "Benzo Fury", see 6-APB.
Benzofuran  1-BenzofuranOther namesCoumarone, benzo[b]furan
1-BenzofuranOther namesCoumarone, benzo[b]furanIdentifiers CAS number 271-89-6 
PubChem 9223 ChemSpider 8868 
UNII LK6946W774 
DrugBank DB04179 KEGG C14512 
ChEBI CHEBI:35260 
ChEMBL CHEMBL363614 
Jmol-3D images Image 1 - o2c1ccccc1cc2
Properties Molecular formula C8H6O Molar mass 118.13 g mol−1 Melting point -18 °C, 255 K, -0 °F
Boiling point 173 °C, 446 K, 343 °F
Hazards LD50 500 mg/kg (mice).[1]  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants.
Contents
Production
Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol.[1]
Laboratory methods
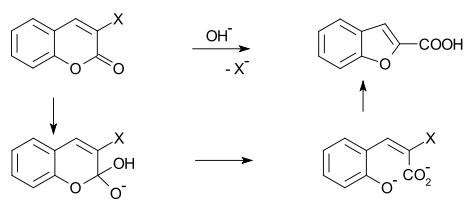
Benzofuran can be prepared by O-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration of the resulting ether.[2] In another method called the "Perkin rearrangement"[3][4] a coumarin is reacted with a hydroxide:
Related compounds
- Furan, an analog without the fused benzene ring.
- Indole, an analog with a nitrogen instead of the oxygen atom.
- Isobenzofuran, the isomer with oxygen in the adjacent position.
- Aurone
- Thunberginol F
References
- ^ a b Gerd Collin, Hartmut Höke "Benzofurans" in Ullmann's Encyclopedia of Industrial Chemistry, 2007, Wiley-VCH, Weinheim. doi: 10.1002/14356007.l03_l01
- ^ Albert W. Burgstahler and Leonard R. Worden “Coumarone” Organic Syntheses, Collected Volume 5, p.251 (1973). http://www.orgsyn.org/orgsyn/pdfs/CV5P0251.pdf
- ^ W. H. Perkin, J. Chem. Soc., 1870, 23, 368; 1871, 24, 37.
- ^ Reactions of carbonyl compounds in basic solutions. Part 32.1 The Perkin rearrangement Keith Bowden and Sinan Battah J. Chem. Soc., Perkin Trans. 2, 1998, 1603 - 1606, doi:10.1039/a801538d
Categories:- Benzofurans
- IARC Group 2B carcinogens
Wikimedia Foundation. 2010.