- Coronary artery bypass surgery
-
"Heart bypass" redirects here. For the technique to take over the function of the heart and lungs during surgery, see Cardiopulmonary bypass.
Coronary artery bypass surgery Intervention 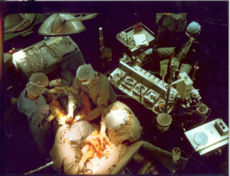
Early in a coronary artery bypass surgery during vein harvesting from the legs (left of image) and the establishment of bypass (placement of the aortic cannula) (bottom of image). The perfusionist and heart-lung machine (HLM) are on the upper right. The patient's head (not seen) is at the bottom.ICD-10-PCS 021209W ICD-9-CM 36.1 MeSH D001026 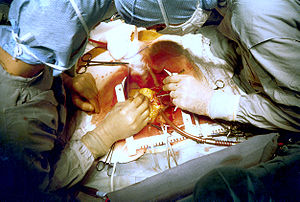 Coronary artery bypass surgery during mobilization (freeing) of the right coronary artery from its surrounding tissue, adipose tissue (yellow). The tube visible at the bottom is the aortic cannula (returns blood from the HLM). The tube above it (obscured by the surgeon on the right) is the venous cannula (receives blood from the body). The patient's heart is stopped and the aorta is cross-clamped. The patient's head (not seen) is at the bottom.
Coronary artery bypass surgery during mobilization (freeing) of the right coronary artery from its surrounding tissue, adipose tissue (yellow). The tube visible at the bottom is the aortic cannula (returns blood from the HLM). The tube above it (obscured by the surgeon on the right) is the venous cannula (receives blood from the body). The patient's heart is stopped and the aorta is cross-clamped. The patient's head (not seen) is at the bottom.
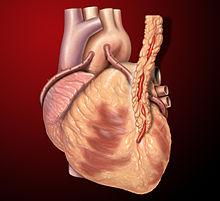 Three coronary artery bypass grafts, a LIMA to LAD and two saphenous vein grafts - one to the right coronary artery (RCA) system and one to the obtuse marginal (OM) system.
Three coronary artery bypass grafts, a LIMA to LAD and two saphenous vein grafts - one to the right coronary artery (RCA) system and one to the obtuse marginal (OM) system.
Coronary artery bypass surgery, also coronary artery bypass graft (CABG pronounced cabbage) surgery, and colloquially heart bypass or bypass surgery is a surgical procedure performed to relieve angina and reduce the risk of death from coronary artery disease. Arteries or veins from elsewhere in the patient's body are grafted to the coronary arteries to bypass atherosclerotic narrowings and improve the blood supply to the coronary circulation supplying the myocardium (heart muscle). This surgery is usually performed with the heart stopped, necessitating the usage of cardiopulmonary bypass; techniques are available to perform CABG on a beating heart, so-called "off-pump" surgery.
Contents
History
The first coronary artery bypass surgery was performed in the United States on May 2, 1960, at the Albert Einstein College of Medicine-Bronx Municipal Hospital Center by a team led by Dr. Robert Goetz and the thoracic surgeon, Dr. Michael Rohman with the assistance of Dr. Jordan Haller and Dr. Ronald Dee.[1][2] In this technique the vessels are held together with circumferential ligatures over an inserted metal ring. The internal mammary artery was used as the donor vessel and was anastomosed to the right coronary artery. The actual anastomosis with the Rosenbach ring took fifteen seconds and did not require cardiopulmonary bypass. The disadvantage of using the internal mammary artery was that, at autopsy nine months later, the anastomosis was open, but an atheromatous plaque had occluded the origin of the internal mammary that was used for the bypass.[citation needed][verification needed]
Russian cardiac surgeon, Dr. Vasilii Kolesov, performed arguably the first successful internal mammary artery–coronary artery anastomosis in 1964.[3][4]
However, Goetz's has been cited by others, including Kolesov,[5] as the first successful human coronary artery bypass.[6][7][8][9][10][11][12] Goetz's case has frequently been overlooked. Confusion has persisted for over 40 years and seems to be due to the absence of a full report and to misunderstanding about the type of anastomosis that was created. The anastomosis was intima-to-intima, with the vessels held together with circumferential ligatures over a specially designed metal ring. Kolesov did the first successful coronary bypass using a standard suture technique in 1964, and over the next five years he performed 33 sutured and mechanically stapled anastomoses in St. Petersburg, Russia.[13][14]
Dr. René Favaloro, an Argentine surgeon, achieved a physiologic approach in the surgical management of coronary artery disease—the bypass grafting procedure—at the Cleveland Clinic in May 1967.[4][15] His new technique used a saphenous vein autograft to replace a stenotic segment of the right coronary artery. Later, he successfully used the saphenous vein as a bypassing channel, which has become the typical bypass graft technique we know today; in the U.S., this vessel is typically harvested endoscopically, using a technique known as endoscopic vessel harvesting (EVH). Soon Dr. Dudley Johnson extended the bypass to include left coronary arterial systems.[4] In 1968, Doctors Charles Bailey, Teruo Hirose and George Green used the internal mammary artery instead of the saphenous vein for the grafting.[4]
Terminology
There are many variations on terminology, in which one or more of "artery", "bypass" or "graft" is left out. The most frequently used acronym for this type of surgery is CABG (pronounced 'cabbage'),[16] pluralized as CABGs (pronounced 'cabbages'). More recently[when?] the term aortocoronary bypass (ACB) has come into popular use. CAGS (Coronary Artery Graft Surgery, pronounced phonetically) should not be confused with coronary angiography (CAG).
Arteriosclerosis is a common arterial disorder characterized by thickening, loss of elasticity, and calcification of arterial walls, resulting in a decreased blood supply.
Atherosclerosis is a common arterial disorder characterized by yellowish plaques of cholesterol, lipids, and cellular debris in the inner layer of the walls of large and medium-sized arteries.
Number of bypasses
The terms single bypass, double bypass, triple bypass, quadruple bypass and quintuple bypass refer to the number of coronary arteries bypassed in the procedure. In other words, a double bypass means two coronary arteries are bypassed (e.g. the left anterior descending (LAD) coronary artery and right coronary artery (RCA)); a triple bypass means three vessels are bypassed (e.g. LAD, RCA, left circumflex artery (LCX)); a quadruple bypass means four vessels are bypassed (e.g. LAD, RCA, LCX, first diagonal artery of the LAD) while quintuple means five. Bypass of more than four coronary arteries is uncommon.
A greater number of bypasses does not imply a person is "more sick", nor does a lesser number imply a person is "healthier."[17] A person with a large amount of coronary artery disease (CAD) may receive fewer bypass grafts owing to the lack of suitable "target" vessels. A coronary artery may be unsuitable for bypass grafting if it is small (< 1 mm or < 1.5 mm depending on surgeon preference), heavily calcified (meaning the artery does not have a section free of CAD) or intramyocardial (the coronary artery is located within the heart muscle rather than on the surface of the heart). Similarly, a person with a single stenosis ("narrowing") of the left main coronary artery requires only two bypasses (to the LAD and the LCX). However, a left main lesion places a person at the highest risk for death from a cardiac cause.[citation needed]
The surgeon reviews the coronary angiogram prior to surgery and identifies the lesions (or "blockages") in the coronary arteries. The surgeon will estimate the number of bypass grafts prior to surgery, but the final decision is made in the operating room upon examination of the heart.
Indications for CABG
Several alternative treatments for coronary artery disease exist. They include:
- Medical management (anti-anginal medications plus statins, antihypertensives, smoking cessation, tight blood sugar control in diabetics)
- Percutaneous coronary intervention (PCI)
Both PCI and CABG are more effective than medical management at relieving symptoms,[18] (e.g. angina, dyspnea, fatigue). CABG is superior to PCI for some patients with multivessel CAD[19][20]
The Surgery or Stent (SoS) trial was a randomized controlled trial that compared CABG to PCI with bare-metal stents. The SoS trial demonstrated CABG is superior to PCI in multivessel coronary disease.[19]
The SYNTAX trial was a randomized controlled trial of 1800 patients with multivessel coronary disease, comparing CABG versus PCI using drug-eluting stents (DES). The study found that rates of major adverse cardiac or cerebrovascular events at 12 months were significantly higher in the DES group (17.8% versus 12.4% for CABG; P=0.002).[20] This was primarily driven by higher need for repeat revascularization procedures in the PCI group with no difference in repeat infarctions or survival. Higher rates of strokes were seen in the CABG group.
The FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus—Optimal Management of Multivessel Disease) trial will compare CABG and DES in patients with diabetes. The registries of the nonrandomized patients screened for these trials may provide as much robust data regarding revascularization outcomes as the randomized analysis.[21]
A study comparing the outcomes of all patients in New York state treated with CABG or percutaneous coronary intervention (PCI) demonstrated CABG was superior to PCI with DES in multivessel (more than one diseased artery) coronary artery disease (CAD). Patients treated with CABG had lower rates of death and of death or myocardial infarction than treatment with a coronary stent. Patients undergoing CABG also had lower rates of repeat revascularization.[22] The New York State registry included all patients undergoing revascularization for coronary artery disease, but was not a randomized trial, and so may have reflected other factors besides the method of coronary revascularization.
The 2004 ACC/AHA CABG guidelines state CABG is the preferred treatment for:[23]
- Disease of the left main coronary artery (LMCA).
- Disease of all three coronary vessels (LAD, LCX and RCA).
- Diffuse disease not amenable to treatment with a PCI.
The 2005 ACC/AHA guidelines further state: CABG is the preferred treatment with other high-risk patients such as those with severe ventricular dysfunction (i.e. low ejection fraction), or diabetes mellitus.[23]
Prognosis
Prognosis following CABG depends on a variety of factors, and successful grafts typically last 8–15 years. In general, CABG improves the chances of survival of patients who are at high risk (generally triple or higher bypass), though statistically after about five years the difference in survival rate between those who have had surgery and those treated by drug therapy diminishes. Age at the time of CABG is critical to the prognosis, younger patients with no complicating diseases doing better, while older patients can usually be expected to suffer further blockage of the coronary arteries.[citation needed]
Controversy
The value of coronary artery bypass surgery in rescuing someone having a heart attack (by immediately alleviating an obstruction) is clearly defined in multiple studies, but studies have failed to find benefit for bypass surgery vs. medical therapy in stable angina patients. The artery bypass can temporarily alleviate chest pain, but does not increase longevity. The "vast majority of heart attacks do not originate with obstructions that narrow arteries".[24]
Loss of mental function is a common complication of bypass surgery, and should influence procedure cost benefit considerations. One published study using MRI imaging just after coronary bypass surgery found significant brain damage in 51% of patients.[25]
Several factors may contribute to immediate cognitive decline. The heart-lung blood circulation system and the surgery itself release a variety of debris, including bits of blood cells, tubing, and plaques. For example, when surgeons clamp and connect the aorta to tubing, resulting emboli block blood flow and cause mini strokes. Other heart surgery factors related to mental damage may be events of hypoxia, high or low body temperature, abnormal blood pressure, irregular heart rhythms, and fever after surgery.[26]
A safer and more permanent and successful way to prevent heart attacks in patients at high risk is to exercise, give up smoking, take "drugs to get blood pressure under control and drive cholesterol levels down to prevent blood clotting".[24] Longer term, behavioral and medication treatment may be the only way to avoid vascular related loss of mental function.[27]
Procedure (simplified)
- The patient is brought to the operating room and moved on to the operating table.
- An anaesthetist places a variety of intravenous lines and injects a painkilling agent (usually fentanyl) followed within minutes by an induction agent (usually propofol) to render the patient unconscious.
- An endotracheal tube is inserted and secured by the anaesthetist or assistant (e.g. respiratory therapist or nurse anaesthetist) and mechanical ventilation is started. General anaesthesia is maintained by a continuous very slow injection of Propofol.
- The chest is opened via a median sternotomy and the heart is examined by the surgeon.
- The bypass grafts are harvested - frequent conduits are the internal thoracic arteries, radial arteries and saphenous veins. When harvesting is done, the patient is given heparin to prevent the blood from clotting.
- In the case of "off-pump" surgery, the surgeon places devices to stabilize the heart.
- If the case is "on-pump", the surgeon sutures cannulae into the heart and instructs the perfusionist to start cardiopulmonary bypass (CPB). Once CPB is established, the surgeon places the aortic cross-clamp across the aorta and instructs the perfusionist to deliver cardioplegia (a special Potassium-mixture, cooled) to stop the heart and slow its metabolism. Usually the patient's machine-circulated blood is cooled to around 84 °F (29 °C)
- One end of each graft is sewn on to the coronary arteries beyond the blockages and the other end is attached to the aorta.
- The heart is restarted; or in "off-pump" surgery, the stabilizing devices are removed. In cases where the aorta is partially occluded by a C-shaped clamp, the heart is restarted and suturing of the grafts to the aorta is done in this partially occluded section of the aorta while the heart is beating.
- Protamine is given to reverse the effects of heparin.
- The sternum is wired together and the incisions are sutured closed.
- The patient is moved to the intensive care unit (ICU) to recover. After awakening and stabilizing in the ICU (approximately one day), the person is transferred to the cardiac surgery ward until ready to go home (approximately four days).
Minimally invasive CABG
Alternate methods of minimally invasive coronary artery bypass surgery have been developed. Off-pump coronary artery bypass (OPCAB) is a technique of performing bypass surgery without the use of cardiopulmonary bypass (the heart-lung machine).[28] Further refinements to OPCAB have resulted in minimally invasive direct coronary artery bypass surgery (MIDCAB), a technique of performing bypass surgery through a 5 to 10 cm incision.[29]
Conduits used for bypass
The choice of conduits is highly dependent upon the particular surgeon and institution. Typically, the left internal thoracic artery (LITA) (previously referred to as left internal mammary artery or LIMA) is grafted to the left anterior descending artery and a combination of other arteries and veins is used for other coronary arteries. The right internal thoracic artery (RITA), the great saphenous vein from the leg and the radial artery from the forearm are frequently used; in the U.S., these vessels are usually harvested endoscopically, using a technique known as endoscopic vessel harvesting (EVH). The right gastroepiploic artery from the stomach is infrequently used given the difficult mobilization from the abdomen.
Graft patency
Grafts can become diseased and may occlude in the months to years after bypass surgery is performed. Patency is a term used to describe the chance that a graft remains open. A graft is considered patent if there is flow through the graft without any significant (>70% diameter) stenosis in the graft.
Graft patency is dependent on a number of factors, including the type of graft used (internal thoracic artery, radial artery, or great saphenous vein), the size or the coronary artery that the graft is anastomosed with, and, of course, the skill of the surgeon(s) performing the procedure. Arterial grafts (e.g. LITA, radial) are far more sensitive to rough handling than the saphenous veins and may go into spasm if handled improperly.
Generally the best patency rates are achieved with the in-situ left internal thoracic artery (the proximal end is left connected to the subclavian artery) with the distal end being anastomosed with the coronary artery (typically the left anterior descending artery or a diagonal branch artery). Lesser patency rates can be expected with radial artery grafts and "free" internal thoracic artery grafts (where the proximal end of the thoracic artery is excised from its origin from the subclavian artery and re-anastomosed with the ascending aorta). Saphenous vein grafts have worse patency rates, but are more available, as the patients can have multiple segments of the saphenous vein used to bypass different arteries.
Veins that are used either have their valves removed or are turned around so that the valves in them do not occlude blood flow in the graft. LITA grafts are longer-lasting than vein grafts, both because the artery is more robust than a vein and because, being already connected to the arterial tree, the LITA need only be grafted at one end. The LITA is usually grafted to the left anterior descending coronary artery (LAD) because of its superior long-term patency when compared to saphenous vein grafts.[30][31]
Sternal precautions
Patients undergoing coronary artery bypass surgery will have to avoid certain things for eight to 12 weeks to reduce the risk of opening the incision. These are called sternal precautions. First, patients need to avoid using their arms excessively, such as pushing themselves out of a chair or reaching back before sitting down. To avoid this, patients are encouraged to build up momentum by rocking several times in their chair before standing up. Second, patients should avoid lifting anything in excess of 5–10 pounds. A gallon (U.S.) of milk weighs approximately 8.5 pounds, and is a good reference point for weight limitations. Finally, patients should avoid overhead activities with their hands, such as reaching for sweaters from the top shelf of a closet or reaching for plates or cups from the cupboard.
Complications
People undergoing coronary artery bypass are at risk for the same complications as any surgery, plus some risks more common with or unique to CABG.
CABG associated
- Postperfusion syndrome (pumphead), a transient neurocognitive impairment associated with cardiopulmonary bypass. Some research shows the incidence is initially decreased by off-pump coronary artery bypass, but with no difference beyond three months after surgery. A neurocognitive decline over time has been demonstrated in people with coronary artery disease regardless of treatment (OPCAB, conventional CABG or medical management). However, a 2009 research study suggests that longer term (over 5 years) cognitive decline is not caused by CABG but is rather a consequence of vascular disease.[32]
- Nonunion of the sternum; internal thoracic artery harvesting devascularizes the sternum increasing risk.
- Myocardial infarction due to embolism, hypoperfusion, or graft failure.
- Late graft stenosis, particularly of saphenous vein grafts due to atherosclerosis causing recurrent angina or myocardial infarction.
- Acute renal failure due to embolism or hypoperfusion.
- Stroke, secondary to embolism or hypoperfusion.
- Vasoplegic syndrome, secondary to cardiopulmonary bypass and hypothermia
- Grafts last 8 – 15 years, and then need to be replaced.
- Pneumothorax: An air collection around the lung that compresses the lung
- Hemothorax: Blood in the space around the lungs
- Pericardial Tamponade: Blood collection around the heart that compresses the heart and causes poor body and brain perfusion. Chest tubes are placed around the heart and lung to prevent this. If the chest tubes become clogged in the early post operative period when bleeding is ongoing this can lead to pericardial tamponade, pneumothorax or hemothorax.
- Pleural Effusion: Fluid in the space around the lungs. This can lead to hypoxia which can slow recovery.
General cardiac surgery
- Post-operative atrial fibrillation: An arrhythmia that sometimes occurs after cardiac surgery.
General surgical
- Infection at incision sites or sepsis.
- Deep vein thrombosis (DVT)
- Anesthetic complications such as malignant hyperthermia.
- Keloid scarring
- Chronic pain at incision sites
- Chronic stress related illnesses
- Death
See also
- Angioplasty
- Cardiothoracic surgery
- Dressler's syndrome
- Hybrid bypass
- Totally endoscopic coronary artery bypass surgery
- Chest tube
References
- ^ Dee, R (2003). "Who assisted whom?". Tex Heart Inst J (Houston: Texas Heart Institute) 30 (1): 90. PMC 152850. PMID 12638685. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=152850.
- ^ Haller, JD; Olearchyk, AS (2002). "Cardiology's 10 greatest discoveries". Tex Heart Inst J (Houston: Texas Heart Institute) 29 (4): 342–4. PMC 140304. PMID 12484626. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=140304.
- ^ Kolessov, VI (October 1967). "Mammary artery-coronary artery anastomosis as method of treatment for angina pectoris". J Thorac Cardiovasc Surg 54 (4): 535–44. PMID 6051440.
- ^ a b c d Mehta, NJ; Khan, IA (2002). "Cardiology's 10 Greatest Discoveries of the 20th Century". Tex Heart Inst J 29 (3): 164–71. PMC 124754. PMID 12224718. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=124754.
- ^ Kolesov, VI; Potashov, LV (1965). "Surgery of coronary arteries" (in Russian). Eksp Khir Anesteziol 10 (2): 3–8. PMID 5851057.
- ^ Olearchyk, AS (1988). "Coronary revascularization: past, present and future". J Ukr Med Assoc North Am 1 (117): 3–34.
- ^ Olearchyk, AS; Olearchyk, RM (January 1999). "Reminiscences of Vasilii I. Kolesov". Ann Thorac Surg 67 (1): 273–6. doi:10.1016/S0003-4975(98)01225-9. PMID 10086577.
- ^ Glenn, WW (April 1972). "Some reflections on the coronary bypass operation". Circulation 45 (4): 869–77. PMID 5016019.
- ^ Ochsner JL, Mills NL (1978). Coronary artery surgery. Philadelphia: Lea & Febiger.
- ^ Cushing, WJ; Magovern, GJ; Olearchyk, AS (November 1986). "Internal mammary artery graft: retrospective report with 17 years' survival". J Thorac Cardiovasc Surg 92 (5): 963–4. PMID 3773554.
- ^ Konstantinov, IE (June 2000). "Robert H. Goetz: the surgeon who performed the first successful clinical coronary artery bypass operation". Ann Thorac Surg 69 (6): 1966–72. doi:10.1016/S0003-4975(00)01264-9. PMID 10892969.
- ^ Konstantinov IE (2000). "Robert H. Goetz: the surgeon who performed the first successful clinical coronary artery bypass operation". Einstein Q J Biol Med 18: 73–8.
- ^ Kolesov, VI; Kolesov, EV (February 1991). "Twenty years' results with internal thoracic artery-coronary artery anastomosis". J Thorac Cardiovasc Surg 101 (2): 360–1. PMID 1992247.
- ^ Haller, JD; Olearchyk, AS (2002). "Cardiology's 10 greatest discoveries". Tex Heart Inst J 29 (4): 342–4. PMC 140304. PMID 12484626. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=140304. "Reference 4"
- ^ Favaloro, RG; Effler, DB; Cheanvechai, C; Quint, RA; Sones Jr, FM (November 1971). "Acute coronary insufficiency (impending myocardial infarction and myocardial infarction): surgical treatment by the saphenous vein graft technique". Am J Cardiol 28 (5): 598–607. doi:10.1016/0002-9149(71)90104-4. PMID 5116978.
- ^ "Bypass Surgery, Coronary Artery". American Heart Association. http://www.americanheart.org/presenter.jhtml?identifier=4484. Retrieved March 26, 2010.
- ^ Ohki, S; Kaneko T; Satoh Y et al. (2002). "[Coronary artery bypass grafting in octogenarian]" (in Japanese). Kyobu geka. the Japanese journal of thoracic surgery 55 (10): 829–33; discussion 833–6. PMID 12233100.
- ^ Rihal C, Raco D, Gersh B, Yusuf S (2003). "Indications for coronary artery bypass surgery and percutaneous coronary intervention in chronic stable angina: review of the evidence and methodological considerations". Circulation 108 (20): 2439–45. doi:10.1161/01.CIR.0000094405.21583.7C. PMID 14623791. http://circ.ahajournals.org/cgi/content/full/108/20/2439.
- ^ a b SoS Investigators (September 28, 2002). "Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the Stent or Surgery trial): a randomised controlled trial". Lancet 360 (9338): 965–70. doi:10.1016/S0140-6736(02)11078-6. PMID 12383664.
- ^ a b Serruys, P.W.; Morice M.-C.; Kappetein A.P. et al. (March 5, 2009). "Percutaneous Coronary Intervention versus Coronary-Artery Bypass Grafting for Severe Coronary Artery Disease". N Engl J Med 360 (10): 961–72. doi:10.1056/NEJMoa0804626. PMID 19228612.
- ^ Desai ND (January 2008). "Pitfalls assessing the role of drug-eluting stents in multivessel coronary disease". Ann Thorac Surg 85 (1): 25–7. doi:10.1016/j.athoracsur.2007.08.063. PMID 18154771.
- ^ Hannan, EL; Wu C; Walford G et al. (January 24, 2008). "Drug-eluting stents vs. coronary-artery bypass grafting in multivessel coronary disease". N. Engl. J. Med. 358 (4): 331–41. doi:10.1056/NEJMoa071804. PMID 18216353.
- ^ a b Eagle, KA; Guyton RA; Davidoff R et al. (October 5, 2004). "ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery)". Circulation 110 (14): e340–437. PMID 15466654.
- ^ a b Kolata, Gina. "New Heart Studies Question the Value Of Opening Arteries" The New York Times, March 21, 2004. Retrieved January 14, 2011.
- ^ Knipp SC, Matatko N, Wilhelm H, Schlamann M, Thielmann M, Lösch C, Diener HC, Jakob H (Mar 2008). "Cognitive outcomes three years after coronary artery bypass surgery: relation to diffusion-weighted magnetic resonance imaging". Ann Thorac Surg. 85 (3): 872–9. doi:10.1016/j.athoracsur.2007.10.083. PMID 18291160.
- ^ Stutz, Bruce "Pumphead: Does the heart-lung machine have a dark side?" Scientific American, January 9, 2009.
- ^ Harmon, Katherine "Heart-Lung Machine May Not Be the Culprit in Post-Op "Pump Head" Syndrome" Scientific American August 6, 2009.
- ^ Sabik, Joseph (2010). "Off Pump Bypass Surgery: Improving outcomes for coronary artery bypass surgery". Clevelandclinic.com. http://my.clevelandclinic.org/heart/disorders/cad/offpump.aspx. Retrieved February 28,2011.
- ^ Sabik, Joseph (2010). "Minimally Invasive Bypass Surgery". Clevelandclinic.com. http://my.clevelandclinic.org/heart/disorders/cad/mini_cabg.aspx. Retrieved February 28,2011.
- ^ Kitamura, S; Kawachi K; Kawata T et al. (March 1996). "[Ten-year survival and cardiac event-free rates in Japanese patients with the left anterior descending artery revascularized with internal thoracic artery or saphenous vein graft: a comparative study]" (in Japanese). Nippon Geka Gakkai Zasshi 97 (3): 202–9. PMID 8649330.
- ^ Arima, M; Kanoh T; Suzuki T et al. (August 2005). "Serial angiographic follow-up beyond 10 years after coronary artery bypass grafting." (PDF). Circ J. 69 (8): 896–902. doi:10.1253/circj.69.896. PMID 16041156. http://www.jstage.jst.go.jp/article/circj/69/8/896/_pdf.
- ^ Harmon, Katherine (August 6, 2009). "Heart-Lung Machine May Not Be the Culprit in Post-Op "Pump Head" Syndrome". ScientificAmerican.com. http://www.scientificamerican.com/article.cfm?id=disease-may-cause-pumphead. Retrieved February 2, 2010.
External links
- A BBC film showing a patient undergoing a double bypass operation.
- Ischemic Heart Disease section in Cardiac Surgey in the Adult
- Coronary Artery Bypass Surgery at NYU Langone Medical Center's Cardiac and Vascular Institute
Categories:
Wikimedia Foundation. 2010.
