- Ibutilide
-
Ibutilide 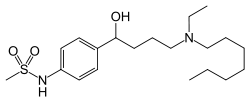
Systematic (IUPAC) name N-(4-{4-[ethyl(heptyl)amino]-1-hydroxybutyl}phenyl)methanesulfonamide Clinical data Trade names Corvert AHFS/Drugs.com monograph MedlinePlus a601248 Pregnancy cat. C Legal status ? Routes Intravenous Pharmacokinetic data Bioavailability N/A Protein binding 40% Metabolism Hepatic oxidation Half-life 6 hours (2-12 hours) Excretion Renal (82%), fecal Identifiers CAS number 122647-32-9 ATC code C01BD05 PubChem CID 60753 DrugBank APRD01025 ChemSpider 54755 
UNII 9L5X4M5L6I 
KEGG D00648 
ChEMBL CHEMBL533 
Chemical data Formula C20H36N2O3S Mol. mass 384.578 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Ibutilide is a Class III antiarrhythmic agent that is indicated for acute cardioconversion of atrial fibrillation and atrial flutter of a recent onset to sinus rhythm. It exerts its antiarrhythmic effect by induction of slow inward sodium current, which prolongs action potential and refractory period (physiology) of myocardial cells. Because of its Class III antiarrhythmic activity, there should not be concomitant administration of Class Ia and Class III agents.
Ibutilide is marketed as Corvert by Pfizer. Administration resulted in successful heart rhythm control in 31-44% of patients within 90 minutes, with sustained polymorphic ventricular tachycardia in 0.9-2.5% of patients. It appears to show better results in atrial flutter as compared to atrial fibrillation.[1]
Contents
Mechanism of action
Unlike most other Class III antiarrhythmic drugs, ibutilide does not produce its prolongation of action potential via blockade of cardiac delayed rectifier of potassium current, nor does it have a sodium-blocking, antiadrenergic, and calcium blocking activity that other Class III agents possess. Thus it is often referred as a “pure” Class III antiarrhythmic drug.
It does have action on the slow sodium channel and promotes the influx of sodium through these slow channels.
Although potassium current seems to play a role, their interactions are complex and not well understood.[2] Ibutilide’s unique mechanism works by an activation of a specific inward sodium current, thus producing its therapeutic response in which a prolonged action potential increases myocytes’ cardiac refractoriness in case of atrial fibrillation and flutter.
Pharmacokinetics
Absorption
Ibutilide is intravenously administered. It has a high first-pass metabolism, which results in a poor bioavailability when taken orally. Individual pharmacokinetic properties are highly viable during the clinical trial.[2][3]
Distribution
Ibutilide has a relatively large volume of distribution among individual subjects, which is about 11L/kg. Approximately 40% of the drug is bound with plasma albumin of healthy volunteers in a trial. This is also approximately close to patients with atrial fibrillation and flutter.[3]
Metabolism
Ibutilide has a high systemic plasma clearance that closes to the hepatic blood flow (29mL/min/kg). Its metabolic pathway is via liver’s cytochrome P450 system by isoenzymes other than CYP3A4 and CYP2D6 by which the heptyl side chain of ibutilide is oxidized.[2][3] With eight metabolites are detected in the urine, however, only one is an active metabolite that shares the similar electrophysiologic property of the Class III antiarrhythmic agents.[2][3][4] The plasma concentration of this metabolite is only less than 10% of ibutilide.[3]
Excretion
After administration of ibutilide, it is quickly excreted by renal pathway with a half-life of approximately 6 hours. Approximately 82% of a 0.01 mg/kg dose is excreted in the urine during the trial. Among those, around 7% is excreted as unchanged drug. The reminder of the drug is excreted in feces (about 19%).[2]
Adverse effects and contraindications
Like other antiarrhythmics, ibutilide can lead to arrhythmia, especially torsades de pointes. Consequently, the drug is contraindicated in patients that are likely to develop arrhythmia; this includes patients that had polymorphic ventricular tachycardia in the past, QT prolongation, sick sinus syndrome, recent myocardial infarction, and others.[5]
Patient Information
This medication will be given intravenously for your heart disease. You will have continuously ECG monitoring during the infusion and 4 hours after your infusion. Some of the minor side effects are headache and irregular heartbeat. If you experience chest pain and respiratory difficulties, you should report to your doctors immediately.[6]
References
- ^ Kowey, PR; Stoenescu, ML (2005). "Selection of drugs in pursuit of a rhythm control strategy". Progress in cardiovascular diseases 48 (2): 139–45. doi:10.1016/j.pcad.2005.06.009. PMID 16253653.
- ^ a b c d e Howard, P.A., Ibutilide: An antiarrhythmic agent for the treatment of atrial fibrillation or flutter. Annals of Pharmacotherapy, 1999. 33(1): p. 38-47. PMID9972384
- ^ a b c d e Pharmacia-Upjohn, Corvert (ibutilide fumarate) injection package insert. July 2002: Kalamazoo, MI.
- ^ Kelly C. Rogers, P., and Douglas A. Wolfe MD, Ibutilide: A class III rapidly acting antidysrhythmic for atrial fibrillation or atrial flutter. Journal of Emergency Medicine January 2001. Volume 20( Issue 1): p. 67-71.
- ^ Jasek, W, ed (2007) (in German). Austria-Codex. 1 (2007/2008 ed.). Vienna: Österreichischer Apothekerverlag. pp. 1768–71. ISBN 3-85200-181-4.
- ^ Lexi-Comp, Lexi-Drugs Online : Ibutilide.
Channel blocker: potassium channel blockers Antiarrhythmic III/delayed rectifier benzofuran (Amiodarone) • quaternary ammonium (Bretylium) • naphthalene (Bunaftine) • phenethylamine (Dofetilide) • sulfonamide (Ibutilide) • pyrimidinone (Nifekalant) • ethanolamine (Sotalol) • cyclopropane (Tedisamil) • E-4031Other/ungrouped/unknown aminopyridines (3,4-Diaminopyridine, 4-Aminopyridine) • indole (Linopirdine, Paxilline) • quaternary ammonium (Tetraethylammonium) • peptide (Maurotoxin, Charybdotoxin)Antiarrhythmic agents (C01B) Channel blockers class Ib (Phase 3←)class Ic (Phase 0→)Amiodarone • Dronedarone • Bretylium • Bunaftine • Dofetilide • Ibutilide • Nifekalant • Sotalol • Tedisamil • Vernakalant • E-4031Receptor agonists
and antagonistsIon transporters Categories:- Antiarrhythmic agents
- Sulfonamides
- Alcohols
- Potassium channel blockers
Wikimedia Foundation. 2010.
