- Dronedarone
-
Dronedarone 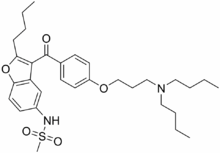
Systematic (IUPAC) name N-(2-Butyl-3-(p-(3-(dibutylamino)propoxy)benzoyl)-
5-benzofuranyl)methanesulfonamideClinical data AHFS/Drugs.com monograph MedlinePlus a609034 Licence data US FDA:link Pregnancy cat. ? Legal status Prescription Routes Oral Pharmacokinetic data Metabolism hepatic Half-life 24 hours Excretion feces Identifiers CAS number 141626-36-0 
ATC code C01BD07 PubChem CID 208898 ChemSpider 180996 
UNII JQZ1L091Y2 
KEGG D02537 
ChEBI CHEBI:50659 
ChEMBL CHEMBL184412 
Chemical data Formula C31H44N2O5S Mol. mass 556.758 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Dronedarone (development codename SR33589 and marketed as Multaq) is a drug by Sanofi-Aventis, mainly for the indication of cardiac arrhythmias. It was approved by the FDA on July 2, 2009. It is now available as 400 mg tablets in 20, 60 and 100 count packages. It is used as an alternative to amiodarone for the treatment of atrial fibrillation and atrial flutter in patients whose hearts have either returned to normal rhythm or who undergo drug therapy or electric shock treatment to maintain normal rhythm.[1] However, the FDA did not approve dronedarone for reducing deaths.[2] A trial of the drug in heart failure was stopped as an interim analysis showed a possible increase in heart failure deaths, in patients with moderate to severe CHF.[3]
In order for dronedarone to be FDA approved the manufacturer had to add a Black box warning, stating that Multaq is contraindicated in patients with NYHA Class IV heart failure, or NYHA Class II–III heart failure with a recent decompensation requiring hospitalization or referral to a specialized heart failure clinic."[4] The FDA alerted healthcare professionals to rare cases of severe liver damage associated with the use of dronedarone.[5]
Contents
Chemistry
Chemically, dronedarone is a benzofuran derivative related to amiodarone, a popular antiarrhythmic the use of which is limited by toxicity due its high iodine content (pulmonary fibrosis, thyroid disease) as well as by liver disease.
In dronedarone, the iodine moieties were removed, to reduce toxic effects on the thyroid and other organs; and a methylsulfonamide group was added, to reduce solubility in fats (lipophilicity) and thus reduce neurotoxic effects. [2] Yet it displays amiodarone-like class III antiarrhythmic activity in vitro[6] and in clinical trials.[3] The drug also appears to exhibit activity in each of the 4 Vaughn-Williams antiarrhythmic classes.[7]
Pharmacokinetics
Dronedarone is less lipophilic than amiodarone, has a much smaller volume of distribution, and has an elimination half-life of 24 hours—this stands in contrast to amiodarone's half-life of several weeks.[8] As a result of these pharmacokinetic characteristics, dronedarone dosing may be less complicated than amiodarone.
Mechanism of action
Dronedarone has been termed a “multichannel blocker” however it is unclear which channel(s) play a pivotal role in its success.[9] Thus dronedarones actions at the cellular level are controversial with most studies suggesting an inhibition in multiple inward potassium currents including rapid delayed rectifier, slow delayed rectifier and Ach-activated inward rectifier.[10] It is also believed to reduce inward rapid Na current and L-type Ca channels. The reduction in K current in some studies was shown to be due to the inhibition of K-Ach channel or associated GTP-binding proteins.[11] Reduction of K+ current by 69% lead to increased AP duration and increased effective refractory periods, thus shown to suppress pacemaker potential of the SA node and return patients to a normal heart rhythm.[12] In a European trial, the average time to recurrence of an arrhythmia was 41 days in the placebo group vs. 96 days in the dronedarone group (similar results obtained in the non-European trial, 59 and 158 days respectively).[13]
Cautions
- NYHA (New York Heart Association) Class IV heart failure or NYHA Class II or III heart failure with a recent decompensation requiring hospitalization or referral to a specialized heart failure clinic.
- Second or third degree atrioventricular (AV) block or sick sinus syndrome (exception in patients with a functional pacemaker)
- Bradycardia less than 50 beats per minute
- QT interval corrected for rate of 500 sec or greater
- PR interval exceeding 280 msec.
- Use of cytochrome P-450 (CYP) 3a isoenzyme inhibitors (includes: clarithromycin, cyclosporine, itraconazole, ketoconazole, nefazodone, ritonavir, telithromycin, voriconazole)
- Use with drugs or herbal supplements that prolong QT interval or increase risk of torsades de points (Class I or III antiarrhythmic agents, phenothiazines, tricyclic antidepressants, certain oral macrolides, ephedra)
- Hepatic impairment. In Jan 2011 the FDA advised about cases of rare, but severe, liver injury, including two cases of acute liver failure leading to liver transplant in patients treated with dronedarone (Multaq). It is not known whether routine periodic monitoring of serum liver enzymes (ALT, AST, and alkaline phosphatase) and bilirubin in patients taking dronedarone will prevent the development of severe liver injury[5].
- Women who are or may become pregnant
- Nursing women
Clinical trials
Clinical trials have compared dronedarone to placebo and to amiodarone, for its ability to reduce atrial fibrillation, to reduce mortality overall and from cardiac causes, and for its adverse effects, including excess mortality.[2] [14] Dronedarone is a non-iodinated class III anti-arrhythmic drug which helps patients return to normal sinus rhythm. This treatment for AF is also known to reduce associated mortality and hospitalizations compared to other similar antiarrhythmic agents.[15]
In the EURIDIS and ADONIS trials in atrial fibrillation (2007), dronedarone was significantly more effective than placebo in maintaining sinus rhythm, with no difference in lung and thyroid function in the short term.[16]
However, in the ANDROMEDA study (2007), dronedarone doubled the death rate compared to placebo, and the trial was halted early.[3] ANDROMEDA enrolled patients with moderate to severe congestive heart failure, a relatively sicker patient population.
In a more recent atrial fibrillation trial, ATHENA, with 4628 subjects, dronedarone was significantly more effective than placebo in reducing the composite endpoint of first hospitalization due to cardiovascular events or death.[17] There was a significant reduction in the rate of cardiovascular death, but not in the rate of death from any cause.[2] Later post-hoc analysis of the ATHENA-results showed a significant reduction in the rate of stroke.[18]
Patients randomized to dronedarone were more likely to develop bradycardia and QT-interval prolongation (but only 1 case of Torsades). Nausea, diarrhea, rash, and creatinine elevation also were more common in the dronedarone arm.
Electro-Cardio conversion results
Multaq has been used by the Veteran's Administration to prepare patients for electro-conversion to sinus rythmn. A patient who had failed conversion 5 times with electro-shock alone, returned to sinus mode after more than 6 years of continuous afibrillation. Doctors prepared for conversion with a ten month loading course of Multaq.[citation needed]
Regulatory review
Originally submitted as a New Drug Application in 2005, dronedarone was reviewed and recommended for approval on March 18, 2009 by an Advisory Committee of the United States Food and Drug Administration (FDA). The FDA is not bound by the Committee's recommendation, but it takes its advice into consideration when reviewing new drug applications.[19] The FDA approved dronedarone on July 2, 2009.
Health Canada was the second major regulatory body to approve the drug, giving its approval on August 12, 2009. The approval is for "treatment of patients with a history of, or current atrial fibrillation to reduce their risk of cardiovascular hospitalization due to this condition." [20]
The European Medicines Agency issued a Summary of Positive Opinion regarding dronedarone on 24 September 2009 recommending to the European Commission to grant a marketing authorization within the European Union.[21]
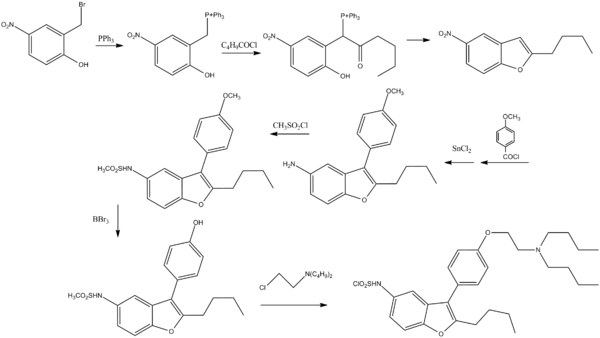
Synthesis
References
- ^ "FDA Approves Multaq to Treat Heart Rhythm Disorder" (Press release). FDA. 2009-07-02. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm170276.htm. Retrieved July 2, 2009.
- ^ a b c d Zimetbaum, PJ (2009). "Dronedarone for atrial fibrillation--an odyssey". The New England journal of medicine 360 (18): 1811–3. doi:10.1056/NEJMp0902248. PMID 19403901. http://content.nejm.org/cgi/content/full/360/18/1811.
- ^ a b c Køber L, Torp-Pedersen C, McMurray JJ et al. (June 2008). "Increased mortality after dronedarone therapy for severe heart failure". N Engl J Med 358 (25): 2678–87. doi:10.1056/NEJMoa0800456. PMID 18565860.
- ^ sanofi aventis
- ^ a b http://www.fda.gov/Drugs/DrugSafety/ucm240011.htm
- ^ Sun W, Sarma JS, Singh BN (30 November 1999). "Electrophysiological effects of dronedarone (SR33589), a noniodinated benzofuran derivative, in the rabbit heart : comparison with amiodarone". Circulation 100 (22): 2276–81. doi:10.1161/01.CIR.100.22.2276. PMID 10578003. http://circ.ahajournals.org/cgi/content/full/100/22/2276.
- ^ http://www.medscape.com/druginfo/monograph?cid=med&drugid=152656&drugname=Multaq+Oral&monotype=monograph&print=1.
- ^ Dale KM, White CM (April 2007). "Dronedarone: an amiodarone analog for the treatment of atrial fibrillation and atrial flutter". Ann Pharmacother 41 (4): 599–605. doi:10.1345/aph.1H524. PMID 17389667.
- ^ Guillemare E, Marion A, Nisato D, Gautier P, “Inhibitory effects of dronedarone on muscarinic K+ current in guinea pig atrial cells,” in Journal of Cardiovascular Pharmacology, 2000 7
- ^ Aimond F, Beck L, Gautier P, Chérif OK, Davy JM, Lorente P, Nisato D, Vassort G, “Cellular and in vivo electrophysiological effects of dronedarone in normal and postmyocardial infarcted rats,” in The Journal of Pharmacology and experimental therapeutics, 2000. 11
- ^ Guillemare E, Marion A, Nisato D, Gautier P, “Inhibitory effects of dronedarone on muscarinic K+ current in guinea pig atrial cells,” in Journal of Cardiovascular Pharmacology, 2000 7
- ^ Aimond F, Beck L, Gautier P, Chérif OK, Davy JM, Lorente P, Nisato D, Vassort G, “Cellular and in vivo electrophysiological effects of dronedarone in normal and postmyocardial infarcted rats,” in The Journal of Pharmacology and experimental therapeutics, 2000. 11
- ^ Singh BN, Connolly SJ, Crijns HJ, Roy D, Kowey PR, Capucci A, Radzik D, Aliot EM, Hohnloser SH; EURIDIS and ADONIS Investigators, “Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter,” in The New England Journal of Medicine, 2007. 12
- ^ Guillemare E, Marion A, Nisato D, Gautier P, “Inhibitory effects of dronedarone on muscarinic K+ current in guinea pig atrial cells,” in Journal of Cardiovascular Pharmacology, 2000. 7
- ^ Connolly SJ, Crijns HJ, Torp-Pedersen C, van Eickels M, Gaudin C, Page RL, Hohnloser SH; ATHENA Investigators, “Analysis of stroke in ATHENA: a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg BID for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter,” in Circulation, 2009.8
- ^ Singh BN, Connolly SJ, Crijns HJ et al. (September 2007). "Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter". N Engl J Med 357 (10): 987–999. doi:10.1056/NEJMoa054686. PMID 17804843. http://content.nejm.org/cgi/content/full/357/10/987.
- ^ Hohnloser SH, Crijns HJ, van Eickels Met al. (February 2009). "Effect of Dronedarone on Cardiovascular Events in Atrial Fibrillation". N Engl J Med 360 (10): 668–678. doi:10.1056/NEJMoa054686. PMID 17804843. http://content.nejm.org/cgi/content/short/360/7/668?query=TOC.
- ^ Connolly SJ, Crijns HJGM, Torp-Pedersen C, van Eyckels M, Gaudin C, Page RL, Hohnloser SH (September 2009). "Analysis of Stroke in ATHENA: A Placebo-Controlled, Double-Blind, Parallel-Arm Trial to Assess the Efficacy of Dronedarone 400 mg BID for the Prevention of Cardiovascular Hospitalization or Death From Any Cause in Patients With Atrial Fibrillation/Atrial Flutter". Circulation 120 (13): 1174–80. doi:10.1161/CIRCULATIONAHA.109.875252. PMID 19752319. http://circ.ahajournals.org/cgi/content/abstract/CIRCULATIONAHA.109.875252v1.
- ^ FDA briefing document on dronedarone
- ^ http://insciences.org/article.php?article_id=6454
- ^ Summary of Positive Opinion (retrieved 1 December 2009)
Antiarrhythmic agents (C01B) Channel blockers Amiodarone • Dronedarone • Bretylium • Bunaftine • Dofetilide • Ibutilide • Nifekalant • Sotalol • Tedisamil • Vernakalant • E-4031Receptor agonists
and antagonistsIon transporters Categories:- Antiarrhythmic agents
- Sulfonamides
- Benzofurans
- Aromatic ketones
- Phenol ethers
Wikimedia Foundation. 2010.