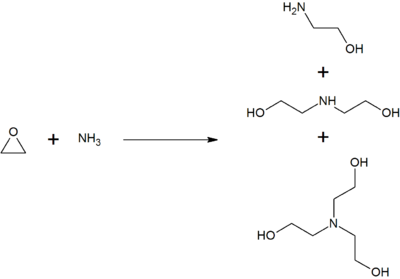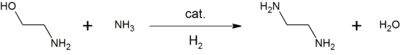- Ethanolamine
-
Ethanolamine 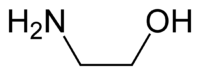
 2-AminoethanolOther names2-Amino-l-Ethanol, Ethanolamine, Monoethanolamine, β-Aminoethanol, β-hydroxyethylamine, β-Aminoethyl alcohol, Glycinol, Olamine, MEA, UN 2491
2-AminoethanolOther names2-Amino-l-Ethanol, Ethanolamine, Monoethanolamine, β-Aminoethanol, β-hydroxyethylamine, β-Aminoethyl alcohol, Glycinol, Olamine, MEA, UN 2491Identifiers CAS number 141-43-5 
PubChem 700 ChemSpider 13835336 
UNII 5KV86114PT 
EC number 205-483-3 DrugBank DB03994 KEGG D05074 
ChEBI CHEBI:16000 
ChEMBL CHEMBL104943 
RTECS number KJ5775000 Jmol-3D images Image 1 - C(CO)N
Properties Molecular formula C2H7NO Molar mass 61.08 g mol−1 Appearance Viscous colourless liquid with ammonia odour Density 1.012 g/cm3 Melting point 10.3 °C, 283 K, 51 °F
Boiling point 170 °C, 443 K, 338 °F
Solubility in water Miscible Vapor pressure 64 Pa (20 °C)[1] Acidity (pKa) 9.50[2] Refractive index (nD) 1.4539 (20 °C)[3] Hazards MSDS JT Baker R-phrases R20, R34, R36/37/38 S-phrases S26, S27, S36/37, S39, S45 NFPA 704 Flash point 85 °C (closed cup) Autoignition
temperature410 °C Explosive limits 5.5 - 17% U.S. Permissible
exposure limit (PEL)3 ppm Related compounds Related compounds N-Methylethanolamine
diethanolamine
triethanolamine (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Ethanolamine, also called 2-aminoethanol or monoethanolamine (often abbreviated as ETA or MEA), is an organic chemical compound that is both a primary amine and a primary alcohol (due to a hydroxyl group). Like other amines, monoethanolamine acts as a weak base. Ethanolamine is a toxic, flammable, corrosive, colorless, viscous liquid with an odor similar to that of ammonia.
Ethanolamine is commonly called monoethanolamine or MEA in order to be distinguished from diethanolamine (DEA) and triethanolamine (TEA). Ethanolamine is the second-most-abundant head group for phospholipids, substances found in biological membranes, and is also used in messenger molecules such as palmitoylethanolamide which has an effect on CB1 receptors.[4]
Contents
Production
Monoethanolamine is produced by reacting ethylene oxide with aqueous ammonia; the reaction also produces diethanolamine and triethanolamine. The ratio of the products can be controlled by changing the stoichiometry of the reactants.[5]
Note that this reaction is exothermic and that controls are needed to prevent a runaway reaction.
Applications
MEA is used in aqueous solutions for scrubbing certain acidic gases. It is used as feedstock in the production of detergents, emulsifiers, polishes, pharmaceuticals, corrosion inhibitors, chemical intermediates.[5][6] For example, reacting ethanolamine with ammonia gives the commonly used chelating agent, ethylenediamine:[5]
In pharmaceutical formulations, MEA is primarily used for buffering or preparation of emulsions.[citation needed]
Gas stream scrubbing
Aqueous solutions of MEA (solutions of MEA in water) are used as a gas stream scrubbing liquid in amine treaters. For example, aqueous MEA is used to remove carbon dioxide (CO2) from flue gas. Aqueous solutions can weakly dissolve certain kinds of gases from a mixed gas stream. The MEA in such solutions, acting as a weak base, then neutralizes acidic compounds dissolved in the solution to turn the molecules into an ionic form, making them polar and considerably more soluble in a cold MEA solution, and thus keeping such acidic gases dissolved in this gas-scrubbing solution. Therefore, large surface area contact with such a cold scrubbing solution in a scrubber unit can selectively remove such acidic components as hydrogen sulfide (H2S) and CO2 from some mixed gas streams. For example, basic solutions such as aqueous MEA or aqueous potassium carbonate can neutralize H2S into hydrosulfide ion (HS-) or CO2 into bicarbonate ion (HCO3-).
H2S and CO2 are only weakly acidic gases. An aqueous solution of a strong base such as sodium hydroxide (NaOH) will not readily release these gases once they have dissolved. However, MEA is rather weak base and will re-release H2S or CO2 when the scrubbing solution is heated. Therefore, the MEA scrubbing solution is recycled through a regeneration unit, which heats the MEA solution from the scrubber unit to release these only slightly acidic gases into a purer form and returns the regenerated MEA solution to the scrubber unit again for reuse.
pH-control amine
Ethanolamine is often used for alkalinization of water in steam cycles of power plants, including nuclear power plants with pressurized water reactors. This alkalinization is performed to control corrosion of metal components. ETA (or sometimes a similar organic amine, e.g., morpholine) is selected because it does not accumulate in steam generators (boilers) and crevices due to its volatility, but rather distributes relatively uniformly throughout the entire steam cycle. In such application, ETA is a key ingredient of so-called "all-volatile treatment" of water (AVT).
References
- ^ "Ethanolamine MSDS". Acros Organics. http://www.paclp.com/content/documents/MSDS/Ethanolamine.pdf.
- ^ Hall, H.K., J. Am. Chem. Soc., 1957, 79, 5441.
- ^ R. E. Reitmeier; V. Sivertz; H. V. Tartar (1940). "Some Properties of Monoethanolamine and its Aqueous Solutions". Journal of the American Chemical Society 62 (8): 1943–1944. doi:10.1021/ja01865a009.
- ^ Calignano, A; La Rana, G; Piomelli, D (2001). "Antinociceptive activity of the endogenous fatty acid amide, palmitylethanolamide". European Journal of Pharmacology 419 (2–3): 191–8. doi:10.1016/S0014-2999(01)00988-8. PMID 11426841.
- ^ a b c Klaus Weissermel, Hans-Jürgen Arpe, Charlet R. Lindley, Stephen Hawkins (2003). "Chap. 7. Oxidation Products of Ethylene". Industrial Organic Chemistry. Wiley-VCH. pp. 159–161. ISBN 3527305785.
- ^ "Ethanolamine". Occupational Safety & Health Administration. http://www.osha.gov/SLTC/healthguidelines/ethanolamine/recognition.html.
External links
Categories:- Alcohols
- Ethylamines
Wikimedia Foundation. 2010.