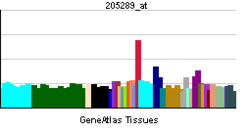
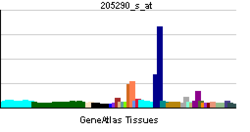
- Bone morphogenetic protein 2
-
Bone morphogenetic protein 2 or BMP-2 belongs to the TGF-β superfamily of proteins.[1]
Contents
Function
BMP-2 like other bone morphogenetic proteins,[2] plays an important role in the development of bone and cartilage. It is involved in the hedgehog pathway, TGF beta signaling pathway, and in cytokine-cytokine receptor interaction. It is involved also in cardiac cell differentiation and epithelial to mesenchymal transition.
BMP-2 and BMP-7 are osteoinductive BMPs: they have been demonstrated to potently induce osteoblast differentiation in a variety of cell types.[3]
Medical uses
Bone morphogenetic protein 2 is shown to stimulate the production of bone.[4] Recombinant human protein (rhBMP-2) is currently available for orthopaedic usage in the United States.[5] Implantation of BMP-2 in a collagen sponge induces new bone formation and can be used for the treatment of bony defects, delayed union, and non-union.[6]
Bone morphogenetic protein 2 has also found its way into the field of Dentistry. Oral Surgery and Implant Dentistry in particular have benefited dramatically from commercially available BMP-2.[7][8][9] The use of dual tapered threaded fusion cages and recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge obtained and maintained intervertebral spinal fusion, improved clinical outcomes, and reduced pain after anterior lumbar interbody arthrodesis in patients with degenerative lumbar disc disease.[10] As an adjuvant to allograft bone or as a replacement for harvested autograft, bone morphogenetic proteins (BMPs) appear to improve fusion rates after spinal arthrodesis in both animal models and humans, while reducing the donor-site morbidity previously associated with such procedures.[11]
Interactions
Bone morphogenetic protein 2 has been shown to interact with BMPR1A.[12][13][14][15]
References
- ^ Sampath TK, Coughlin JE, Whetstone RM, Banach D, Corbett C, Ridge RJ, Ozkaynak E, Oppermann H, Rueger DC (5 August 1990). "Bovine osteogenic protein is composed of dimers of OP-1 and BMP-2A, two members of the transforming growth factor-beta superfamily". J. Biol. Chem. 265 (22): 13198–205. PMID 2376592. http://www.jbc.org/cgi/content/abstract/265/22/13198.
- ^ Chen D, Zhao M, Mundy GR (December 2004). "Bone morphogenetic proteins". Growth Factors 22 (4): 233–41. doi:10.1080/08977190412331279890. PMID 15621726.
- ^ Marie PJ, Debiais F, Haÿ E (2002). "Regulation of human cranial osteoblast phenotype by FGF-2, FGFR-2 and BMP-2 signaling". Histol. Histopathol. 17 (3): 877–85. PMID 12168799. http://www.hh.um.es/Abstracts/Vol_17/17_3/17_3_877.htm.
- ^ Urist, Marshall R. (1965). "Bone: formation by autoinduction". Science 12:150 (698): 893–899. doi:10.1126/science.150.3698.893. PMID 5319761.
- ^ Khan SN, Lane JM (May 2004). "The use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in orthopaedic applications". Expert Opin Biol Ther 4 (5): 741–8. doi:10.1517/14712598.4.5.741. PMID 15155165.
- ^ Geiger M, Li RH, Friess W (November 2003). "Collagen sponges for bone regeneration with rhBMP-2". Adv. Drug Deliv. Rev. 55 (12): 1613–29. doi:10.1016/j.addr.2003.08.010. PMID 14623404.
- ^ Allegrini S, Yoshimoto M, Salles MB, König B (February 2004). "Bone regeneration in rabbit sinus lifting associated with bovine BMP". J. Biomed. Mater. Res. Part B Appl. Biomater. 68 (2): 127–31. doi:10.1002/jbm.b.20006. PMID 14737759.
- ^ Schlegel KA, Thorwarth M, Plesinac A, Wiltfang J, Rupprecht S (December 2006). "Expression of bone matrix proteins during the osseus healing of topical conditioned implants: an experimental study". Clin Oral Implants Res 17 (6): 666–72. doi:10.1111/j.1600-0501.2006.01214.x. PMID 17092225.
- ^ Schliephake H, Aref A, Scharnweber D, Bierbaum S, Roessler S, Sewing A (October 2005). "Effect of immobilized bone morphogenic protein 2 coating of titanium implants on peri-implant bone formation". Clin Oral Implants Res 16 (5): 563–9. doi:10.1111/j.1600-0501.2005.01143.x. PMID 16164462.
- ^ Burkus JK, Gornet MF, Schuler TC, Kleeman TJ, Zdeblick TA (May 2009). "Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2". J Bone Joint Surg Am 91 (5): 1181–9. doi:10.2106/JBJS.G.01485. PMID 19411467.
- ^ Subach BR, Haid RW, Rodts GE, Kaiser MG (2001). "Bone morphogenetic protein in spinal fusion: overview and clinical update". Neurosurg Focus 10 (4): E3. PMID 16732630.
- ^ Nickel, J; Dreyer M K, Kirsch T, Sebald W (2001). "The crystal structure of the BMP-2:BMPR-IA complex and the generation of BMP-2 antagonists". The Journal of bone and joint surgery. American volume (United States) 83-A Suppl 1 (Pt 1): S7–14. ISSN 0021-9355. PMID 11263668.
- ^ Kirsch, T; Nickel J, Sebald W (Feb. 2000). "Isolation of recombinant BMP receptor IA ectodomain and its 2:1 complex with BMP-2". FEBS Lett. (NETHERLANDS) 468 (2-3): 215–9. doi:10.1016/S0014-5793(00)01214-X. ISSN 0014-5793. PMID 10692589.
- ^ Kirsch, T; Nickel J, Sebald W (Jul. 2000). "BMP-2 antagonists emerge from alterations in the low-affinity binding epitope for receptor BMPR-II". EMBO J. (ENGLAND) 19 (13): 3314–24. doi:10.1093/emboj/19.13.3314. ISSN 0261-4189. PMC 313944. PMID 10880444. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=313944.
- ^ Gilboa, L; Nohe A, Geissendörfer T, Sebald W, Henis Y I, Knaus P (Mar. 2000). "Bone morphogenetic protein receptor complexes on the surface of live cells: a new oligomerization mode for serine/threonine kinase receptors". Mol. Biol. Cell (UNITED STATES) 11 (3): 1023–35. ISSN 1059-1524. PMC 14828. PMID 10712517. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=14828.
Further reading
- Nickel J, Dreyer MK, Kirsch T, Sebald W (2001). "The crystal structure of the BMP-2:BMPR-IA complex and the generation of BMP-2 antagonists.". The Journal of bone and joint surgery. American volume 83-A Suppl 1 (Pt 1): S7–14. PMID 11263668.
- Kawamura C, Kizaki M, Ikeda Y (2003). "Bone morphogenetic protein (BMP)-2 induces apoptosis in human myeloma cells.". Leuk. Lymphoma 43 (3): 635–9. doi:10.1080/10428190290012182. PMID 12002771.
- Marie PJ, Debiais F, Haÿ E (2003). "Regulation of human cranial osteoblast phenotype by FGF-2, FGFR-2 and BMP-2 signaling.". Histol. Histopathol. 17 (3): 877–85. PMID 12168799.
PDB gallery 1es7: COMPLEX BETWEEN BMP-2 AND TWO BMP RECEPTOR IA ECTODOMAINS1reu: Structure of the bone morphogenetic protein 2 mutant L51P1rew: Structural refinement of the complex of bone morphogenetic protein 2 and its type IA receptor2goo: Ternary Complex of BMP-2 bound to BMPR-Ia-ECD and ActRII-ECD2h62: Crystal structure of a ternary ligand-receptor complex of BMP-22h64: Crystal structure of a ternary ligand-receptor complex of BMP-23bmp: HUMAN BONE MORPHOGENETIC PROTEIN-2 (BMP-2)External links
Cell signaling: TGF beta signaling pathway TGF beta superfamily of ligands TGF beta family (TGF-β1, TGF-β2, TGF-β3)
Bone morphogenetic proteins (BMP2, BMP3, BMP4, BMP5, BMP6, BMP7, BMP8a, BMP8b, BMP10 , BMP15)
Growth differentiation factors (GDF1, GDF2, GDF3, GDF5, GDF6, GDF7, Myostatin/GDF8, GDF9, GDF10, GDF11, GDF15)
Other (Activin and inhibin, Anti-müllerian hormone, Nodal)TGF beta receptors
(Activin, BMP)TGFBR1: Activin type 1 receptors (ACVR1, ACVR1B, ACVR1C) · ACVRL1 · BMPR1 (BMPR1A · BMPR1B)
TGFBR2: Activin type 2 receptors (ACVR2A, ACVR2B) · AMHR2 · BMPR2
TGFBR3: betaglycanTransducers/SMAD Ligand inhibitors Coreceptors Other SARACategories:- Human proteins
- Developmental genes and proteins
- Bone morphogenetic protein
- TGFβ domain
Wikimedia Foundation. 2010.