- Kidney stone
-
Kidney stone Classification and external resources 
A kidney stone, 8 millimeters (0.31 in) in diameterICD-10 N20.0 – N20.9 ICD-9 592.0, 592.1, 592.9 DiseasesDB 11346 MedlinePlus 000458 eMedicine med/1600 MeSH D007669 A kidney stone, also known as a renal calculus (from the Latin ren, "kidney" and calculus, "pebble") is a solid concretion or crystal aggregation formed in the kidneys from dietary minerals in the urine. Urinary stones are typically classified by their location in the kidney (nephrolithiasis), ureter (ureterolithiasis), or bladder (cystolithiasis), or by their chemical composition (calcium-containing, struvite, uric acid, or other compounds). Kidney stones are a significant source of morbidity. 80% of those with kidney stones are men. Men most commonly experience their first episode between age 30–40 years, while for women the age at first presentation is somewhat later.
Kidney stones typically leave the body by passage in the urine stream, and many stones are formed and passed without causing symptoms. If stones grow to sufficient size (usually at least 3 millimeters (0.12 in)) they can cause obstruction of the ureter. Ureteral obstruction causes postrenal azotemia and hydronephrosis (distension and dilation of the renal pelvis and calyces), as well as spasm of the ureter. This leads to pain, most commonly felt in the flank (the area between the ribs and hip), lower abdomen and groin (a condition called renal colic). Renal colic can be associated with nausea, vomiting, fever, blood in the urine, pus in the urine, and painful urination. Renal colic typically comes in waves lasting 20 – 60 minutes, beginning in the flank or lower back and often radiating to the groin or genitals. The diagnosis of kidney stones is made on the basis of information obtained from the history, physical examination, urinalysis, and radiographic studies. Ultrasound examination and blood tests may also aid in the diagnosis.
When a stone causes no symptoms, watchful waiting is a valid option. For symptomatic stones, pain control is usually the first measure, using medications such as non-steroidal anti-inflammatory drugs (NSAIDs) or opioids. More severe cases may require surgical intervention. For example, some stones can be shattered into smaller fragments using extracorporeal shock wave lithotripsy (ESWL). Some cases require more invasive forms of surgery. Examples of these are cystoscopic procedures such as laser lithotripsy, or percutaneous techniques such as percutaneous nephrolithotomy. Sometimes, a tube (ureteral stent) may be placed in the ureter to bypass the obstruction and alleviate the symptoms, as well as to prevent ureteral stricture after ureteroscopic stone removal.
Contents
Signs and symptoms
 Diagram showing the typical location of renal colic, from below the rib cage to just above the pelvis
Diagram showing the typical location of renal colic, from below the rib cage to just above the pelvis
The hallmark of stones that obstruct the ureter or renal pelvis is excruciating intermittent pain that radiates from the flank to the groin or to the genital area and inner thigh.[1] This particular type of pain, known as renal colic, is often described as one of the strongest pain sensations known.[2] Renal colic caused by kidney stones is commonly accompanied by urinary urgency, restlessness, blood in the urine, sweating, nausea and vomiting. It typically comes in waves lasting 20 – 60 minutes caused by peristaltic contractions of the ureter as it attempts to expel the stone.[1] The embryological link between the urinary tract, the genital system and the gastrointestinal tract is the basis of the radiation of pain to the gonads, as well as the nausea and vomiting that are also common in urolithiasis.[3] Postrenal azotemia and hydronephrosis can be observed following the obstruction of urine flow through one or both ureters.[4]
Causes
Dietary factors that increase the risk of stone formation include low fluid intake, and high dietary intake of animal protein, sodium, refined sugars, fructose and high fructose corn syrup[5], oxalate, grapefruit juice, apple juice, and cola drinks.[6]
Calcium
Calcium is one component of the most common type of human kidney stones, calcium oxalate. Some studies suggest that people who take supplemental calcium have a higher risk of developing kidney stones, and these findings have been used as the basis for setting the recommended daily intake (RDI) for calcium in adults.[7] In the Women's Health Initiative, postmenopausal women who consumed 1,000 milligrams (15 gr) of supplemental calcium and 400 international units of vitamin D per day for 7 years had a 17% higher risk of developing kidney stones than subjects taking a placebo.[8] The Nurses' Health Study also showed an association between supplemental calcium intake and kidney stone formation.[6]
Unlike supplemental calcium, high intakes of dietary calcium do not appear to cause kidney stones and may actually protect against their development.[6][8] This is perhaps related to the role of calcium in binding ingested oxalate in the gastrointestinal tract. As the amount of calcium intake decreases, the amount of oxalate available for absorption into the bloodstream increases; this oxalate is then excreted in greater amounts into the urine by the kidneys. In the urine, oxalate is a very strong promoter of calcium oxalate precipitation, about 15 times stronger than calcium. In fact, current evidence suggests that the consumption of diets low in calcium is associated with a higher overall risk for the development of kidney stones.[9] For most individuals however, other risk factors for kidney stones such as high intakes of dietary oxalates and low fluid intake probably play a greater role than that of calcium intake.[10]
Other electrolytes
Aside from calcium, other electrolytes appear to influence the formation of kidney stones. For example, by increasing urinary calcium excretion, high dietary sodium may increase the risk of stone formation.[6] Fluoridation of drinking water may increase the risk of kidney stone formation by a similar mechanism, though further epidemiologic studies are warranted to determine whether fluoride in drinking water is associated with an increased incidence of kidney stones.[11] On the other hand, high dietary intake of potassium appears to reduce the risk of stone formation because potassium promotes the urinary excretion of citrate, an inhibitor of urinary crystal formation. High dietary intake of magnesium also appears to reduce the risk of stone formation somewhat, because like citrate, magnesium is also an inhibitor of urinary crystal formation.[6]
Animal protein
Diets in Western nations typically contain more animal protein than the body needs. Urinary excretion of excess sulfurous amino acids (e.g., cysteine and methionine), uric acid and other acidic metabolites from animal protein acidifies the urine, which promotes the formation of kidney stones. The body often balances this acidic urinary pH by leaching calcium from the bones, which further promotes the formation of kidney stones. Low urinary citrate excretion is also commonly found in those with a high dietary intake of animal protein, whereas vegetarians tend to have higher levels of citrate excretion.[6]
Vitamins
Despite a widely-held belief in the medical community that ingestion of vitamin C supplements is associated with an increased incidence of kidney stones,[12] the evidence for a causal relationship between vitamin C supplements and kidney stones is inconclusive. While excess dietary intake of vitamin C might increase the risk of calcium oxalate stone formation, in practice this is rarely encountered. The link between vitamin D intake and kidney stones is also tenuous. Excessive vitamin D supplementation may increase the risk of stone formation by increasing the intestinal absorption of calcium, but there is no evidence that correction of vitamin D deficiency increases the risk of stone formation.[6]
Other
There are no conclusive data demonstrating a cause and effect relationship between alcohol consumption and kidney stones. However, some have theorized that certain behaviors associated with frequent and binge drinking can lead to systemic dehydration, which can in turn lead to the development of kidney stones.[13] The American Urological Association has projected that increasing global temperatures will lead to an increased incidence of kidney stones in the United States by expanding the "kidney stone belt" of the southern United States.[14]
Pathophysiology
Supersaturation of urine
When the urine becomes supersaturated (when the urine solvent contains more solutes than it can hold in solution) with one or more calculogenic (crystal-forming) substances, a seed crystal may form through the process of nucleation.[15] Heterogeneous nucleation (where there is a solid surface present on which a crystal can grow) proceeds more rapidly than homogeneous nucleation (where a crystal must grow in liquid medium with no such surface), because it requires less energy. Adhering to cells on the surface of a renal papilla, a seed crystal can grow and aggregate into an organized mass. Depending on the chemical composition of the crystal, the stone-forming process may proceed more rapidly when the urine pH is unusually high or low.[16]
Supersaturation of the urine with respect to a calculogenic compound is pH-dependent. For example, at a pH of 7.0, the solubility of uric acid in urine is 158 milligrams/100 milliliters. Reducing the pH to 5.0 decreases the solubility of uric acid to less than 8 milligrams/100 milliliters. One can readily see that the formation of uric acid stones requires a combination of hyperuricosuria (high urine uric acid levels) and low urine pH; hyperuricosuria alone is not associated with uric acid stone formation if the urine pH is alkaline.[17] Supersaturation of the urine is a necessary but not a sufficient condition for the development of any urinary calculus.[15] Supersaturation is likely the underlying cause of uric acid and cystine stones, but calcium-based stones (especially calcium oxalate stones) may have a more complex etiology.[18]
Inhibitors of stone formation
Normal urine contains chelating agents such as citrate that inhibit the nucleation, growth, and aggregation of calcium-containing crystals. Other endogenous inhibitors include calgranulin (an S-100 calcium binding protein), Tamm-Horsfall protein (THP), glycosaminoglycans, uropontin (a form of osteopontin), nephrocalcin (an acidic glycoprotein), prothrombin F1 peptide, and bikunin (uronic acid-rich protein). The biochemical mechanisms of action of these substances have not yet been thoroughly elucidated. However, when these substances fall below their normal proportions, stones can form out of an aggregation of crystals.[19]
Kidney stones often result from a combination of factors, rather than a single, well-defined cause. Stones are more common in people whose diet is very high in animal protein or who do not consume enough water or calcium.[1] They can result from an underlying metabolic condition, such as distal renal tubular acidosis,[20] Dent's disease,[21] hyperparathyroidism,[22] primary hyperoxaluria[23] or medullary sponge kidney. In fact, studies show that 3% to 20% of people who form kidney stones have medullary sponge kidney.[15][24] Kidney stones are also more common in people with Crohn's disease.[25] People with recurrent kidney stones are often screened for these disorders. This is typically done with a 24–hour urine collection that is chemically analyzed for deficiencies and excesses that promote stone formation.[4]
Diagnosis
Diagnosis of kidney stones is made on the basis of information obtained from the history, physical examination, urinalysis, and radiographic studies.[26] Clinical diagnosis is usually made on the basis of the location and severity of the pain, which is typically colicky in nature (comes and goes in spasmodic waves). Pain in the back occurs when calculi produce an obstruction in the kidney.[27] Physical examination may reveal fever and tenderness at the costovertebral angle on the affected side.[26]
Imaging studies
Calcium-containing stones are relatively radiodense, and they can often be detected by a traditional radiograph of the abdomen that includes the kidneys, ureters, and bladder (KUB film).[28] Some 60% of all renal stones are radiopaque.[29][30] In general, calcium phosphate stones have the greatest density, followed by calcium oxalate and magnesium ammonium phosphate stones. Cystine calculi are only faintly radiodense, while uric acid stones are usually entirely radiolucent.[31]
Where available, a noncontrast helical CT scan with 5 millimeters (0.20 in) sections is the diagnostic modality of choice in the radiographic evaluation of suspected nephrolithiasis.[3][26][29][32][33] All stones are detectable on CT scans except very rare stones composed of certain drug residues in the urine,[28] such as from indinavir.
Where CT scan is unavailable, an intravenous pyelogram (IVP) may be performed to help confirm the diagnosis of urolithiasis. The IVP involves intravenous injection of a contrast agent followed by a KUB film. Uroliths present in the kidneys, ureters or bladder may be better defined by the use of this contrast agent. Stones can also be detected by a retrograde pyelogram, where a similar contrast agent is injected directly into the distal ostium of the ureter (where the ureter terminates as it enters the bladder).[29]
Ultrasound imaging of the kidneys can sometimes be useful as it gives details about the presence of hydronephrosis, suggesting the stone is blocking the outflow of urine.[28] Radiolucent stones, which do not appear on CT scans, may show up on ultrasound imaging studies. Other advantages of renal ultrasonography include its low cost and absence of radiation exposure. Ultrasound imaging is useful for detecting stones in situations where x-rays or CT scans are discouraged, such as in children or pregnant women.[34] Despite these advantages, renal ultrasonography is not currently considered a substitute for noncontrast helical CT scan in the initial diagnostic evaluation of urolithiasis.[32] The main reason for this is that compared with CT, renal ultrasonography more often fails to detect small stones (especially ureteral stones) as well as other serious disorders that could be causing the symptoms.[1]
Laboratory examination
Laboratory investigations typically carried out include:[26][28][32][35]
- microscopic examination of the urine, which may show red blood cells, bacteria, leukocytes, urinary casts and crystals;
- urine culture to identify any infecting organisms present in the urinary tract and sensitivity to determine the susceptibility of these organisms to specific antibiotics;
- complete blood count (CBC), looking for neutrophilia (increased neutrophil granulocyte count) suggestive of bacterial infection, as seen in the setting of struvite stones;
- renal function tests to look for abnormally high blood calcium blood levels (hypercalcemia);
- 24 hour urine collection to measure total daily urinary volume, magnesium, sodium, uric acid, calcium, citrate, oxalate and phosphate;
- collection of stones (by urinating through a StoneScreen kidney stone collection cup or a simple tea strainer) is useful. Chemical analysis of collected stones can establish their composition, which in turn can help to guide future preventive and therapeutic management.
Classification
Kidney stones are typically classified by their location and their chemical composition.
Chemical composition
Calcium-containing stones
By far the most common type of kidney stones worldwide are those that contain calcium. For example, calcium-containing stones represent about 80% of all cases in the United States; these typically contain calcium oxalate either alone or in combination with calcium phosphate in the form of apatite or brushite.[15][19] Factors that promote the precipitation of oxalate crystals in the urine, such as primary hyperoxaluria, are associated with the development of calcium oxalate stones.[23] The formation of calcium phosphate stones is associated with conditions such as hyperparathyroidism[22] and renal tubular acidosis.[36]
Struvite stones
About 10–15% of urinary calculi are composed of struvite (ammonium magnesium phosphate, NH4MgPO4·6H2O).[37] Struvite stones (also known as "infection stones", urease or triple-phosphate stones), form most often in the presence of infection by urea-splitting bacteria. Using the enzyme urease, these organisms metabolize urea into ammonia and carbon dioxide. This alkalinizes the urine, resulting in favorable conditions for the formation of struvite stones. Proteus mirabilis, Proteus vulgaris and Morganella morganii are the most common organisms isolated; less common organisms include Ureaplasma urealyticum, and some species of Providencia, Klebsiella, Serratia, andEnterobacter. These infection stones are commonly observed in people who have factors that predispose them to urinary tract infections, such as those with spinal cord injury and other forms of neurogenic bladder, ileal conduit urinary diversion, vesicoureteral reflux, and obstructive uropathies. They are also commonly seen in people with underlying metabolic disorders, such as idiopathic hypercalciuria, hyperparathyroidism, and gout. Infection stones can grow rapidly, forming large calyceal staghorn (antler-shaped) calculi requiring invasive surgery such as percutaneous nephrolithotomy for definitive treatment.[37]
Uric acid stones
About 5–10% of all stones are formed from uric acid.[20] People with certain metabolic abnormalities, including obesity,[6] may produce uric acid stones. Uric acid stones may form in association with conditions that cause hyperuricosuria (an excessive amount of uric acid in the urine) with or without hyperuricemia (an excessive amount of uric acid in the serum). They may also form in association with disorders of acid/base metabolism where the urine is excessively acidic (low pH), resulting in precipitation of uric acid crystals. A diagnosis of uric acid urolithiasis is supported by the presence of a radiolucent stone in the face of persistent urine acidity, in conjunction with the finding of uric acid crystals in fresh urine samples.[38]
Other types
People with certain rare inborn errors of metabolism have a propensity to accumulate crystal-forming substances in their urine. For example, those with cystinuria, cystinosis, and Fanconi syndrome may form stones composed of cystine. People afflicted with xanthinuria often produce stones composed of xanthine. People afflicted with adenine phosphoribosyltransferase deficiency may produce 2,8-dihydroxyadenine stones,[39] alkaptonurics produce homogentisic acid stones, and iminoglycinurics produce stones of glycine, proline and hydroxyproline.[40][41] Urolithiasis has also been noted to occur in the setting of therapeutic drug use, with crystals of drug forming within the renal tract in some people currently being treated with agents such as indinavir,[42] sulfadiazine[43] and triamterene.[44]
Location
 Radiograph showing a large staghorn calculus involving the major calyces and renal pelvis in a person with severe scoliosis. Struvite stones can grow rapidly, forming large calyceal staghorn calculi that can require invasive surgery such as percutaneous nephrolithotomy or even anatrophic nephrolithotomy for definitive treatment.
Radiograph showing a large staghorn calculus involving the major calyces and renal pelvis in a person with severe scoliosis. Struvite stones can grow rapidly, forming large calyceal staghorn calculi that can require invasive surgery such as percutaneous nephrolithotomy or even anatrophic nephrolithotomy for definitive treatment.
Urolithiasis refers to stones originating anywhere in the urinary system, including the kidneys and bladder.[3] Nephrolithiasis (from the Greek νεφρός (nephros, "kidney") and λίθoς (lithos, "stone")) refers to the presence of such calculi in the kidneys. Calyceal) calculi refers to aggregations in either the minor or major calyx, parts of the kidney that pass urine into the ureter (the tube connecting the kidneys to the urinary bladder). The condition is called ureterolithiasis when a calculus or calculi are located in the ureter. Stones may also form or pass into the bladder, a condition referred to as cystolithiasis.[45]
Prevention
Dietary measures
Specific therapy should be tailored to the type of stones involved. Diet can have a profound influence on the development of kidney stones. Preventive strategies include some combination of dietary modifications and medications with the goal of reducing the excretory load of calculogenic compounds on the kidneys.[9][46][47] Current dietary recommendations to minimize the formation of kidney stones include:[48]
- Increasing fluid intake of citrate-rich fluids (especially citrate-rich fluids such as lemonade and orange juice), with the objective of increasing urine output to more than 2 liters per day
- Attempt to maintain a calcium (Ca) intake of 1000 – 1200 mg per day
- Limiting sodium (Na) intake to less than 2300 mg per day
- Limiting vitamin C intake to less than 1000 mg per day (A positive association between animal protein consumption and recurrence of kidney stones has been shown in men, but not yet in women.[49])
- Limiting animal protein intake to no more than 2 meals daily, with less than 170 – 230 gram per day
- Limiting consumption of foods containing high amounts of oxalate (such as spinach, strawberries, nuts, rhubarb, wheat germ, dark chocolate, cocoa, brewed tea)
Maintenance of dilute urine by means of vigorous fluid therapy is beneficial in all forms of nephrolithiasis, so increasing urine volume is a key principle for the prevention of kidney stones. Fluid intake should be sufficient to maintain a urine output of at least 2 liters (68 US fl oz) per day.[49] A high fluid intake has been associated with a 40% reduction in recurrence risk.[32]
Calcium binds with available oxalate in the gastrointestinal tract, thereby preventing its absorption into the bloodstream, and reducing oxalate absorption decreases kidney stone risk in susceptible people.[50] Because of this, some nephrologists and urologists recommend chewing calcium tablets during meals containing oxalate foods.[51] Calcium citrate supplements can be taken with meals if dietary calcium cannot be increased by other means. The preferred calcium supplement for people at risk of stone formation is calcium citrate because it helps to increase urinary citrate excretion.[47]
Aside from vigorous oral hydration and consumption of more dietary calcium, other prevention strategies include avoidance of large doses of supplemental vitamin C and restriction of oxalate-rich foods such as leaf vegetables, rhubarb, soy products and chocolate.[52] However, no randomized controlled trial of oxalate restriction has yet been performed to test the hypothesis that oxalate restriction reduces the incidence of stone formation.[51] There is some evidence that magnesium (Mg) intake decreases the risk of symptomatic nephrolithiasis.[52]
Urine alkalinization
The mainstay for medical management of uric acid stones is alkalinization (increasing the pH) of the urine. Uric acid stones are among the few types amenable to dissolution therapy, referred to as chemolysis. Chemolysis is usually achieved through the use of oral medications, although in some cases intravenous agents or even instillation of certain irrigating agents directly onto the stone can be performed, using antegrade nephrostomy or retrograde ureteral catheters.[17] Acetazolamide (Diamox) is a medication that alkalinizes the urine. In addition to acetazolamide or as an alternative, certain dietary supplements are available that produce a similar alkalinization of the urine. These include sodium bicarbonate, potassium citrate, magnesium citrate, and Bicitra (a combination of citric acid monohydrate and sodium citrate dihydrate). Aside from alkalinization of the urine, these supplements have the added advantage of increasing the urinary citrate level, which helps to reduce the aggregation of calcium oxalate stones.[17]
Increasing the urine pH to around 6.5 provides optimal conditions for dissolution of uric acid stones. Increasing the urine pH to a value higher than 7.0 increases the risk of calcium phosphate stone formation. Testing the urine periodically with nitrazine paper can help to ensure that the urine pH remains in this optimal range. Using this approach, stone dissolution rate can be expected to be around 10 millimeters (0.39 in) of stone radius per month.[17]
Diuretics
One of the recognized medical therapies for prevention of stones is the thiazide and thiazide-like diuretics, such as chlorthalidone or indapamide. These drugs inhibit the formation of calcium-containing stones by reducing urinary calcium excretion.[1] Sodium restriction is necessary for clinical effect of thiazides, as sodium excess promotes calcium excretion. Thiazides work best for renal leak hypercalciuria (high urine calcium levels), a condition in which high urinary calcium levels are caused by a primary kidney defect. Thiazides are useful for treating absorptive hypercalciuria, a condition in which high urinary calcium is a result of excess absorption from the gastrointestinal tract.[19]
Allopurinol
For people with hyperuricosuria and calcium stones, allopurinol is one of the few treatments that has been shown to reduce kidney stone recurrences. Allopurinol interferes with the production of uric acid in the liver. The drug is also used in people with gout or hyperuricemia (high serum uric acid levels).[53] Dosage is adjusted to maintain a reduced urinary excretion of uric acid. Serum uric acid level at or below 6 milligrams/100 milliliters) is often a therapeutic goal. Hyperuricemia (high serum uric acid levels) is not necessary for the formation of uric acid stones; hyperuricosuria can occur in the presence of normal or even low serum uric acid. Some practitioners advocate adding allopurinol only in people in whom hyperuricosuria and hyperuricemia persists despite the use of a urine alkalinizing agent such as sodium bicarbonate or potassium citrate.[17]
Management
Medical
Stone size influences the rate of spontaneous stone passage. For example, up to 98% of small stones (less than 5 millimeters (0.20 in) in diameter) may pass spontaneously through urination within four weeks of the onset of symptoms,[33] but for larger stones (5 to 10 millimeters (0.20 to 0.39 in) in diameter), the rate of spontaneous passage decreases to less than 53%.[54] Initial stone location also influences the likelihood of spontaneous stone passage. Spontaneous passage rates increase from 48% for stones located in the proximal ureter to 79% for stones located at the vesico-ureteric junction, regardless of stone size.[54] Assuming there is no high-grade obstruction or associated infection in the urinary tract, and symptoms are relatively mild, various non-surgical measures can be used to encourage the passage of a stone.[17] Repeat stone formers benefit from more intense management, including proper fluid intake and use of certain medications. In addition, it is also clear that careful surveillance is required in order to maximize the clinical course for people who are stone formers.[55]
Analgesia
Management of pain often requires intravenous administration of NSAIDs or opioids.[1] Orally-administered medications are often effective for less severe discomfort. Intravenous acetaminophen also appears to be effective.[34]
Expulsion therapy
The use of medications to speed the spontaneous passage of ureteral calculi is referred to as medical expulsive therapy.[56] Several agents including alpha adrenergic blockers (such as tamsulosin) and calcium channel blockers (such as nifedipine) have been found to be effective.[56] A combination of tamsulosin and a corticosteroid may be better than tamsulosin alone.[56] These treatments also appears to be a useful adjunct to lithotripsy.[33]
Surgical
 A lithotriptor machine in an operating room. Other equipment is seen in the background, including an anesthesia machine and a mobile fluoroscopic system (or "C-arm").
A lithotriptor machine in an operating room. Other equipment is seen in the background, including an anesthesia machine and a mobile fluoroscopic system (or "C-arm").
Most stones under 5 millimeters (0.20 in) pass spontaneously.[9][33] Prompt surgery may, nonetheless, be required with persons with only one working kidney, bilateral obstructing stones, a urinary tract infection and thus, it is presumed, an infected kidney, or intractable pain.[57] Beginning in the mid-1980s, less invasive treatments such as Extracorporeal shock wave lithotripsy (ESWL), ureteroscopy, and percutaneous nephrolithotomy began to replace open surgery as the modalities of choice for the surgical management of urolithiasis.[33]
Extracorporeal shock wave lithotripsy
Extracorporeal shock wave lithotripsy (ESWL) involves the use of a lithotriptor machine to deliver externally-applied, focused, high-intensity pulses of ultrasonic energy to cause fragmentation of a stone over a period of around 30–60 minutes. Following its introduction in United States in February 1984, ESWL was rapidly and widely accepted as a treatment alternative for renal and ureteral stones.[58] ESWL is currently used in the treatment of uncomplicated stones located in the kidney and upper ureter, provided the aggregate stone burden (stone size and number) is less than 20 millimeters (0.79 in) and the anatomy of the involved kidney is normal.[59][60] In fact, some 80 – 85% of simple renal calculi can be effectively treated with shock wave lithotripsy.[33] A number of factors can influence the efficacy of ESWL, including chemical composition of the stone, presence of anomalous renal anatomy and the specific location of the stone within the kidney, presence of hydronephrosis, body mass index, and distance of the stone from the surface of the skin.[58] Common adverse effects of ESWL include acute trauma such as bruising at the site of shock administration and damage to blood vessels of the kidney.[61][62] In fact, the vast majority of people who are treated with a typical dose of shock waves using currently accepted treatment settings are likely to experience some degree of acute kidney injury.[58] ESWL-induced acute kidney injury is dose-dependent (increases with the total number of shock waves administered and with the power setting of the lithotriptor) and can be severe,[58] including internal bleeding and subcapsular hematomas. On rare occasions, such cases may require blood transfusion and even lead to acute renal failure. Hematoma rates may be related to the type of lithotriptor used; hematoma rates of less than 1% and up to 13% have been reported for different lithotriptor machines.[62] Recent studies show reduced acute tissue injury when the treatment protocol includes a brief pause following the initiation of treatment, and both improved stone breakage and a reduction in injury when ESWL is carried out at slow shock wave rate.[58]
In addition to the aforementioned potential for acute kidney injury, animal studies suggest that these acute injuries may progress to scar formation, resulting in loss of functional renal volume.[61][62] Recent prospective studies also indicate that elderly people are at increased risk of developing new-onset hypertension following ESWL. In addition, a retrospective case-control study published by researchers from the Mayo Clinic in 2006 has found an increased risk of developing diabetes mellitus and hypertension in people who had undergone ESWL, compared with age and gender-matched people who had undergone non-surgical treatment. Whether or not acute trauma progresses to long-term effects probably depends on multiple factors that include the shock wave dose (i.e., the number of shock waves delivered, rate of delivery, power setting, acoustic characteristics of the particular lithotriptor, and frequency of retreatment) as well as certain intrinsic predisposing pathophysiologic risk factors.[58]
To address these concerns, the American Urological Association established the Shock Wave Lithotripsy Task Force in order to provide expert opinion on the safety and risk-benefit ratio of ESWL. The task force published a white paper outlining their conclusions in 2009. The task force concluded that the risk-benefit ratio remains favorable for many people.[58] The advantages of ESWL include its noninvasive nature, the fact that it is technically easy to treat most upper urinary tract calculi, and that, at least acutely, it is a well-tolerated, low-morbidity treatment for the vast majority of people. However, they recommended slowing the shock wave firing rate from 120 pulses per minute to 60 pulses per minute to reduce the risk of renal injury and increases the degree of stone fragmentation.[58]
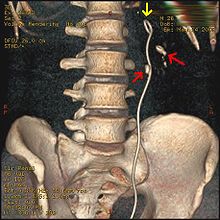
Ureteroscopic surgery
Three-dimensional reconstructed CT scan image of a ureteral stent in the left kidney (indicated by yellow arrow). There is a kidney stone in the inferior renal pelvis (highest red arrow) and one in the ureter beside the stent (lower red arrow)Ureteroscopy has become increasingly popular as flexible and rigid fiberoptic ureteroscopes have become smaller. One ureteroscopic technique involves the placement of a ureteral stent (a small tube extending from the bladder, up the ureter and into the kidney) to provide immediate relief of an obstructed kidney. Stent placement can be useful for saving a kidney at risk for postrenal acute renal failure due to the increased hydrostatic pressure, swelling and infection (pyelonephritis and pyonephrosis) caused by an obstructing stone. Ureteral stents vary in length from 24 to 30 centimeters (9.4 to 12 in) and most have a shape commonly referred to as a "double-J" or "double pigtail", because of the curl at both ends. They are designed to allow urine to flow past an obstruction in the ureter. They may be retained in the ureter for days to weeks as infections resolve and as stones are dissolved or fragmented by ESWL or by some other treatment. The stents dilate the ureters, which can facilitate instrumentation, and they also provide a clear landmark to aid in the visualization of the ureters and any associated stones on radiographic examinations. The presence of indwelling ureteral stents may cause minimal to moderate discomfort, frequency or urgency incontinence, and infection, which in general resolves on removal. Most ureteral stents can be removed cystoscopically during an office visit under topical anesthesia after resolution of the urolithiasis.[63]
More definitive ureteroscopic techniques for stone extraction (rather than simply bypassing the obstruction) include basket extraction and ultrasound ureterolithotripsy. Laser lithotripsy is another technique, which involves the use of a holmium:yttrium aluminium garnet (Ho:YAG) laser to fragment stones in the bladder, ureters and kidneys.[64]
Ureteroscopic techniques are generally more effective than ESWL for treating stones located in the lower ureter, with success rates of 93–100% using Ho:YAG laser lithotripsy.[54] Although ESWL has been traditionally preferred by many practitioners for treating stones located in the upper ureter, more recent experience suggests that ureteroscopic offers distinct advantages in the treatment of upper ureteral stones. Specifically, the overall success rate is higher, there is less need for repeat interventions and postoperative visits, and treatment costs are lower after ureteroscopic treatment when compared with ESWL. These advantages are especially apparent with stones greater than 10 millimeters (0.39 in) in diameter. However, because ureteroscopy of the upper ureter is much more challenging than ESWL, many urologists still prefer to use ESWL as a first-line treatment for stones of less than 10 millimeters (0.39 in) and ureteroscopy for those greater than 10 millimeters (0.39 in) in diameter.[54] Ureteroscopy is the preferred treatment in pregnant and morbidly obese people, as well as those with bleeding disorders.[33]
More invasive operations
Percutaneous nephrolithotomy or, rarely, anatrophic nephrolithotomy is the treatment of choice for large or complicated stones (such as calyceal staghorn calculi) or stones that cannot be extracted using less invasive procedures.[26][33]
Epidemiology
Urolithiasis is a significant source of morbidity, affecting all geographical, cultural, and racial groups. The lifetime risk is about 10 – 15% in the developed world, but can be as high as 20 – 25% in the Middle East. The increased risk of dehydration in hot climates, coupled with a diet that is 50% lower in calcium and 250% higher in oxalates compared to Western diets, accounts for the higher net risk in the Middle East.[65] Although one might expect more calcium oxalate stones, uric acid stones are actually more common in the Middle East than calcium-containing stones.[15]
In North America and Europe, the annual incidence (number of new cases per year) of kidney stones is roughly 0.5%. In the United States, the prevalence (frequency in the population) of urolithiasis has increased from 3.2% to 5.2% from the mid-1970s to the mid-1990s.[20] The total cost for treating urolithiasis was US$2 billion in 2003.[28] Eighty percent (80%) of those with kidney stones are men; most stones in women are due to either metabolic defects (such as cystinuria) or infection.[37] Men most commonly experience their first episode between age 30 – 40 years, whereas for women the age at first presentation is somewhat later.[37] The age of onset shows a bimodal distribution in women, with episodes peaking at 35 and 55 years.[28] Recurrence rates are estimated at 50% over a 10 year period and 75% over 20 years,[20] with some people experiencing ten or more episodes over the course of a lifetime.[37]
History
Portrait of Jan de Doot, by Carel van Savoyen, holding the kidney stone he removed from himself according to a 1652 account in the book Observationes Medicae by Nicolaes Tulp See also: List of kidney stone formers
See also: List of kidney stone formersThe existence of kidney stones was first recorded thousands of years ago, and lithotomy for the removal of stones is one of the earliest known surgical procedures.[66] In 1901, a stone was discovered in the pelvis of an ancient Egyptian mummy, and was dated to 4,800 BC. Medical texts from ancient Mesopotamia, India, China, Persia, Greece, and Rome all mentioned calculous disease. Part of the Hippocratic Oath suggests that there were practicing surgeons in Ancient Greece to whom physicians were to defer for lithotomies. The Roman medical treatise De Medicina by Aulus Cornelius Celsus contained a description of lithotomy,[67] and this work served as the basis for this procedure up until the 18th century.[68]
Famous people who were kidney stone formers include Napoleon I, Napoleon III, Peter the Great, Louis XIV, George IV, Oliver Cromwell, Lyndon B. Johnson, Benjamin Franklin, Michel de Montaigne, Francis Bacon, Isaac Newton, Samuel Pepys, William Harvey, Herman Boerhaave, and Antonio Scarpa.[69]
New techniques in lithotomy began to emerge starting in 1520, but the operation remained risky. It was only after Henry Jacob Bigelow popularized the technique of litholapaxy in 1878[70] that the mortality rate dropped from about 24% down to 2.4%. However, other treatment techniques were developed that continued to produce a high level of mortality, especially among inexperienced urologists.[68][69] In 1980, Dornier MedTech introduced extracorporeal shock wave lithotripsy for breaking up stones via acoustical pulses, and this technique has since come into widespread use.[58]
Research directions
Crystallization of calcium oxalate appears to be inhibited by certain substances in the urine that retard the formation, growth, aggregation, and adherence of calcium oxalate crystals to renal cells. By purifying urine using salt precipitation, isoelectric focusing, and size-exclusion chromatography, some researchers have found that calgranulin, a protein formed in the kidney, is a potent inhibitor of the in vivo formation of calcium oxalate crystals. Considering its extremely high levels of inhibition of growth and aggregation of calcium oxalate crystals, calgranulin might be an important intrinsic factor in the prevention of nephrolithiasis.[71]
See also
Footnotes
- ^ a b c d e f Preminger, GM (2007). "Chapter 148: Stones in the Urinary Tract". In Cutler, RE. The Merck Manual of Medical Information Home Edition (3rd ed.). Whitehouse Station, New Jersey: Merck Sharp and Dohme Corporation. http://www.merckmanuals.com/home/sec12/ch148/ch148a.html.
- ^ Wolf Jr. JS (2011). "Background". Nephrolithiasis. New York: WebMD. http://emedicine.medscape.com/article/437096-overview. Retrieved 2011-07-27.
- ^ a b c Pearle, MS; Calhoun, EA; Curhan, GC (2007). "Chapter 8: Urolithiasis". In Litwin, MS; Saigal, CS. Urologic Diseases in America (NIH Publication No. 07–5512). Bethesda, Maryland: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, United States Public Health Service, United States Department of Health and Human Services. pp. 283–319. http://kidney.niddk.nih.gov/statistics/uda/Urologic_Diseases_in_America.pdf.
- ^ a b Cavendish, M (2008). "Kidney disorders". Diseases and Disorders. 2 (1st ed.). Tarrytown, New York: Marshall Cavendish Corporation. pp. 490–3. ISBN 9780761477723. http://books.google.com/?id=L5fGm_7ThKEC&pg=PA507&lpg=PA507&dq=0761477721#v=onepage&q&f=false.
- ^ Knight J, Assimos DG, Easter L, Holmes RP (2010). "Metabolism of fructose to oxalate and glycolate". http://www.ncbi.nlm.nih.gov/pubmed/20842614.
- ^ a b c d e f g h Johri, N; Cooper B, Robertson W, Choong S, Rickards D, Unwin R (2010). "An update and practical guide to renal stone management". Nephron Clinical Practice 116 (3): c159–71. doi:10.1159/000317196. PMID 20606476. http://content.karger.com/produktedb/produkte.asp?typ=fulltext&file=000317196.
- ^ Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Summary, pp. 1-14 in Committee to Review Dietary Reference Intakes for Vitamin D and Calcium (2011)
- ^ a b Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Tolerable upper intake levels: calcium and vitamin D, pp. 403-56 in Committee to Review Dietary Reference Intakes for Vitamin D and Calcium (2011)
- ^ a b c Parmar, MS (2004). "Kidney stones". British Medical Journal 328 (7453): 1420–4. doi:10.1136/bmj.328.7453.1420. PMC 421787. PMID 15191979. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=421787.
- ^ Liebman, M; Al-Wahsh, IA (2011). "Probiotics and Other Key Determinants of Dietary Oxalate Absorption". Advances in Nutrition 2 (May): 254–60. doi:10.3945/an.111.000414. http://advances.nutrition.org/content/2/3/254.full.pdf. Retrieved 2011-07-27.
- ^ Committee on Fluoride in Drinking Water of the National Academy of Sciences (2006). "Chapter 9: Effects on the Renal System". Fluoride in Drinking Water: A Scientific Review of EPA's Standards. Washington, DC: The National Academies Press. pp. 236–48. ISBN 0-309-65799-7. http://www.nap.edu/openbook.php?record_id=11571&page=268.
- ^ Goodwin, JS; Mangum, MR (1998). "Battling quackery: attitudes about micronutrient supplements in American academic medicine". Archives of Internal Medicine 158 (20): 2187–91. doi:10.1001/archinte.158.20.2187. PMID 9818798.
- ^ Rodman, JS; Seidman, C (1996). "Chapter 8: Dietary Troublemakers". In Rodman, JS; Seidman, C; Jones, R. No More Kidney Stones (1st ed.). New York: John Wiley & Sons, Inc.. pp. 46–57. ISBN 9780471125877.
- ^ Brawer, MK; Makarov, DV; Partin, AW; Roehrborn, CG; Nickel, JC; Lu, SH; Yoshimura, N; Chancellor, MB et al. (2008). "Best of the 2008 AUA Annual Meeting: Highlights from the 2008 Annual Meeting of the American Urological Association, May 17–22, 2008, Orlando, FL". Reviews in Urology 10 (2): 136–56. PMC 2483319. PMID 18660856. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2483319.
- ^ a b c d e Reilly Jr. RF, Chapter 13: Nephrolithiasis, pp. 192–207 in Reilly Jr. and Perazella (2005)
- ^ Perazella MA, Chapter 14: Urinalysis, pp. 209–26 in Reilly Jr. and Perazella (2005)
- ^ a b c d e f Knudsen BE, Beiko DT and Denstedt JD, Chapter 16: Uric Acid Urolithiasis, pp. 299-308 in Stoller and Meng (2007)
- ^ Wolf Jr. JS (2011). "Pathophysiology: formation of stones". Nephrolithiasis. New York: WebMD. http://emedicine.medscape.com/article/437096-overview#a0104. Retrieved 2011-07-27.
- ^ a b c Coe, FL; Evan, A; Worcester, E (2005). "Kidney stone disease". The Journal of Clinical Investigation 115 (10): 2598–608. doi:10.1172/JCI26662. PMC 1236703. PMID 16200192. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1236703.
- ^ a b c d Moe, OW (2006). "Kidney stones: pathophysiology and medical management". The Lancet 367 (9507): 333–44. doi:10.1016/S0140-6736(06)68071-9. PMID 16443041. http://emed.chris-barton.com/PDF/kidney%20stones%20pathophys%20and%20rx.pdf.
- ^ Thakker, RV (2000). "Pathogenesis of Dent's disease and related syndromes of X-linked nephrolithiasis". Kidney International 57 (3): 787–93. doi:10.1046/j.1523-1755.2000.00916.x. PMID 10720930. http://www.nature.com/ki/journal/v57/n3/pdf/4491399a.pdf.
- ^ a b National Endocrine and Metabolic Diseases Information Service (2006). "Hyperparathyroidism (NIH Publication No. 6–3425)". Information about Endocrine and Metabolic Diseases: A-Z list of Topics and Titles. Bethesda, Maryland: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Public Health Service, US Department of Health and Human Services. http://www.endocrine.niddk.nih.gov/pubs/hyper/hyper.htm. Retrieved 2011-07-27.
- ^ a b Hoppe, B; Langman, CB (2003). "A United States survey on diagnosis, treatment, and outcome of primary hyperoxaluria". Pediatric Nephrology 18 (10): 986–91. doi:10.1007/s00467-003-1234-x. PMID 12920626.
- ^ National Kidney and Urologic Diseases Information Clearinghouse (2008). "Medullary Sponge Kidney (NIH Publication No. 08–6235)". Kidney & Urologic Diseases: A-Z list of Topics and Titles. Bethesda, Maryland: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Public Health Service, US Department of Health and Human Services. http://kidney.niddk.nih.gov/kudiseases/pubs/medullaryspongekidney/. Retrieved 2011-07-27.
- ^ National Digestive Diseases Information Clearinghouse (2006). "Crohn's Disease (NIH Publication No. 06–3410)". Digestive Diseases: A-Z List of Topics and Titles. Bethesda, Maryland: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, United States Public Health Service, United States Department of Health and Human Services. http://digestive.niddk.nih.gov/ddiseases/pubs/crohns/. Retrieved 2011-07-27.
- ^ a b c d e Anoia, EJ; Paik, ML; Resnick, MI (2009). "Chapter 7: Anatrophic Nephrolithomy". In Graham, SD; Keane, TE. Glenn's Urologic Surgery (7th ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 45–50. ISBN 9780781791410. http://books.google.com/?id=GahMzaKgMKAC&pg=PA45&lpg=PA45&dq=nephrolithiasis+diagnosis+radiographic#v=onepage&q=nephrolithiasis%20diagnosis%20radiographic&f=false.
- ^ Weaver SH, Jenkins P (2002). "Chapter 14: Renal and Urological Care". Illustrated Manual of Nursing Practice (3rd ed.). Lippincott Williams & Wilkins. ISBN 1582550824.
- ^ a b c d e f Pietrow, PK; Karellas ME (2006). "Medical Management of Common Urinary Calculi". American Family Physician 74 (1): 86–94. PMID 16848382. http://www.aafp.org/afp/2006/0701/p86.pdf.
- ^ a b c Smith, RC; Varanelli, M (2000). "Diagnosis and Management of Acute Ureterolithiasis: CT Is Truth". American Journal of Roentgenology 175 (1): 3–6. PMID 10882237. http://www.ajronline.org/cgi/reprint/175/1/3.pdf.
- ^ Bushinsky, D; Coe, FL; Moe, OW (2007). "Chapter 37: Nephrolithiasis". In Brenner, BM. Brenner and Rector's The Kidney. 1 (8th ed.). Philadelphia: WB Saunders. pp. 1299–349. ISBN 9781416031055. http://www.expertconsultbook.com/expertconsult/op/book.do?method=display&type=bookPage&decorator=none&eid=4-u1.0-B978-1-4160-3105-5..50039-6&isbn=978-1-4160-3105-5#lpState=open&lpTab=contentsTab&content=4-u1.0-B978-1-4160-3105-5..50039-6%3Bfrom%3Dtoc%3Btype%3DbookPage%3Bisbn%3D978-1-4160-3105-5&search=none.
- ^ Smith, RC; Levine JA, Rosenfeld AT (1999). "Helical CT of urinary tract stones. Epidemiology, origin, pathophysiology, diagnosis, and management". Radiologic Clinics of North America 37 (5): 911–52, v. doi:10.1016/S0033-8389(05)70138-X. PMID 10494278.
- ^ a b c d Fang, LST (2009). "Chapter 135: Approach to the Paient with Nephrolithiasis". In Goroll, AH; Mulley, AG. Primary care medicine: office evaluation and management of the adult patient (6th ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 962–7. ISBN 978071775137. http://books.google.com/?id=bIZvJPcSEXMC&pg=PA964&lpg=PA964&dq=nephrolithiasis+%22physical+examination%22#v=onepage&q=nephrolithiasis%20%22physical%20examination%22&f=false.
- ^ a b c d e f g h Miller, NL; Lingeman, JE (2007). "Management of kidney stones". BMJ 334 (7591): 468–72. doi:10.1136/bmj.39113.480185.80. PMC 1808123. PMID 17332586. http://www.bmj.com/content/334/7591/468.full.pdf.
- ^ a b Cormier, CM; Canzoneri BJ, Lewis DF, Briery C, Knoepp L, Mailhes JB (2006). "Urolithiasis in Pregnancy: Current Diagnosis, Treatment, and Pregnancy Complications". Obstetrical and Gynecological Survey 61 (11): 733–41. doi:10.1097/01.ogx.0000243773.05916.7a. PMID 17044950. http://www.utilis.net/Morning%20Topics/Obstetrics/Kidney%20stones.pdf.
- ^ National Kidney and Urologic Diseases Information Clearinghouse (2007). "Kidney Stones in Adults (NIH Publication No. 08–2495)". Kidney & Urologic Diseases: A-Z list of Topics and Titles. Bethesda, Maryland: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Public Health Service, US Department of Health and Human Services. http://kidney.niddk.nih.gov/kudiseases/pubs/stonesadults/. Retrieved 2011-07-27.
- ^ National Endocrine and Metabolic Diseases Information Service (2008). "Renal Tubular Acidosis (NIH Publication No. 09–4696)". Kidney & Urologic Diseases: A-Z list of Topics and Titles. Bethesda, Maryland: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Public Health Service, US Department of Health and Human Services. http://kidney.niddk.nih.gov/kudiseases/pubs/tubularacidosis/. Retrieved 2011-07-27.
- ^ a b c d e Weiss, M; Liapis, H; Tomaszewski, JE; Arend, LJ (2007). "Chapter 22: Pyelonephritis and other infections, reflux nephropathy, hydronephrosis, and nephrolithiasis". In Jennette, JC; Olson, JL; Schwartz, MM et al.. Heptinstall's Pathology of the Kidney. 2 (6th ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 991–1082. ISBN 9780781747509. http://books.google.com/?id=oWymx2hp1OoC&pg=PA886&lpg=PA886&dq=%22AA+amyloidosis%22+pyelonephritis#v=onepage&q=%22AA%20amyloidosis%22%20pyelonephritis&f=false.
- ^ Halabe, A; Sperling, O (1994). "Uric acid nephrolithiasis". Mineral and Electrolyte Metabolism 20 (6): 424–31. ISSN 0378-0392. PMID 7783706.
- ^ Kamatani, N (1996). "Adenine phosphoribosyltransferase (APRT) deficiency" (in Japanese). Nippon Rinsho. Japanese Journal of Clinical Medicine 54 (12): 3321–7. ISSN 0047-1852. PMID 8976113.
- ^ Rosenberg, LE; Durant JL, Elsas LJ (1968). "Familial iminoglycinuria. An inborn error of renal tubular transport". The New England Journal of Medicine 278 (26): 1407–13. doi:10.1056/NEJM196806272782601. PMID 5652624.
- ^ Coşkun T, Ozalp I, Tokatli A (1993). "Iminoglycinuria: a benign type of inherited aminoaciduria". The Turkish Journal of Pediatrics 35 (2): 121–5. ISSN 0041-4301. PMID 7504361.
- ^ Merck Sharp & Dohme Corporation (2010). "Patient Information about Crixivan for HIV (Human Immunodeficiency Virus) Infection". Crixivan® (indinavir sulfate) Capsules. Whitehouse Station, New Jersey: Merck Sharp & Dohme Corporation. http://www.merck.com/product/usa/pi_circulars/c/crixivan/crixivan_ppi.pdf. Retrieved 2011-07-27.
- ^ Schlossberg, D; Samuel, R (2011). "Sulfadiazine". Antibiotic Manual: A Guide to Commonly Used Antimicrobials (1st ed.). Shelton, Connecticut: People's Medical Publishing House. pp. 411–12. ISBN 9781607950844. http://books.google.com/books/about/Antibiotic_Manual.html?id=sCXn0xOVKNoC.
- ^ Carr, MC; Prien EL Jr, Babayan RK (1990). "Triamterene nephrolithiasis: renewed attention is warranted". Journal of Urology 144 (6): 1339–40. PMID 2231920.
- ^ McNutt, WF (1893). "Chapter VII: Vesical Calculi (Cysto-lithiasis)". Diseases of the kidneys and bladder: a text-book for students of medicine. IV: Diseases of the Bladder. Philadelphia: J.B. Lippincott Company. pp. 185–6. http://books.google.com/?id=RP0oAAAAYAAJ&pg=PA185&lpg=PA185&dq=cystolithiasis+symptoms#v=onepage&q&f=false.
- ^ Goldfarb, DS; Coe, FL (1999). "Prevention of recurrent nephrolithiasis". American Family Physician 60 (8): 2269–76. PMID 10593318. http://www.aafp.org/afp/991115ap/2269.html.
- ^ a b Finkielstein, VA; Goldfarb, DS (2006). "Strategies for preventing calcium oxalate stones". Canadian Medical Association Journal 174 (10): 1407–9. doi:10.1503/cmaj.051517. PMC 1455427. PMID 16682705. http://www.cmaj.ca/cgi/reprint/174/10/1407.
- ^ Paterson, R; Fernandez, A; Razvi, H; Sutton, R (2010). "Evaluation and medical management of the kidney stone patient". Canadian Urological Association Journal 4 (6): 375–9. PMC 2997825. PMID 21191493. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2997825.
- ^ a b Taylor, EN; Curhan, GC (2006 Sep). "Diet and fluid prescription in stone disease.". Kidney international 70 (5): 835–9. PMID 16837923.
- ^ Heaney, RP (2006). "Nutrition and Chronic Disease". Mayo Clinic Proceedings 81 (3): 297–9. doi:10.4065/81.3.297. PMID 16529131. http://www.mayoclinicproceedings.com/content/81/3/297.full.pdf. Retrieved 2011-07-27.
- ^ a b Tiselius, HG (2003). "Epidemiology and medical management of stone disease". British Journal of Urology International 91 (8): 758–67. doi:10.1046/j.1464-410X.2003.04208.x. PMID 12709088.
- ^ a b Taylor, EN; Stampfer, MJ; Curhan, GC (2004). "Dietary Factors and the Risk of Incident Kidney Stones in Men: New Insights after 14 Years of Follow-up". Journal of the American Society of Nephrology 15 (12): 3225–32. doi:10.1097/01.ASN.0000146012.44570.20. PMID 15579526. http://jasn.asnjournals.org/content/15/12/3225.full.pdf.
- ^ Cameron, JS; Simmonds, HA (1987). "Use and abuse of allopurinol". British Medical Journal 294 (6586): 1504–5. doi:10.1136/bmj.294.6586.1504. PMC 1246665. PMID 3607420. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1246665.
- ^ a b c d Gettman, MT; Segura, JW (2005). "Management of ureteric stones: issues and controversies". British Journal of Urology International 95 (Supplement 2): 85–93. doi:10.1111/j.1464-410X.2005.05206.x. PMID 15720341.
- ^ Macaluso, JN (1999). "Management of stone disease—bearing the burden". The Journal of Urology 156 (5): 1579–80. doi:10.1016/S0022-5347(01)65452-1. PMID 8863542.
- ^ a b c Seitz, C; Liatsikos, E, Porpiglia, F, Tiselius, HG, Zwergel, U (2009 Sep). "Medical therapy to facilitate the passage of stones: what is the evidence?". European urology 56 (3): 455–71. PMID 19560860.
- ^ Young JG and Keeley FX, Chapter 38: Indications for Surgical Removal, Including Asymptomatic Stones, pp. 441-54 in Rao, Preminger and Kavanagh (2011)
- ^ a b c d e f g h i Shock Wave Lithotripsy Task Force (2009). "Current Perspective on Adverse Effects in Shock Wave Lithotripsy". Clinical Guidelines. Linthicum, Maryland: American Urological Association. http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/whitepaper.pdf. Retrieved 2011-07-27.
- ^ Lingeman, J.E., Matlaga, B.R., and Evan, A.P. (2007). Surgical management of urinary lithiasis. In:Campbell-Walsh Urology Edited by AJ Wein, LR Kavoussi, AC Novick, AW Partin, CA Peters. Philadelphia: W. B. Saunders.(pp. 1431-1507)
- ^ Preminger, GM; Tiselius, HG; Assimos, DG; Alken, P; Buck, AC; Gallucci, M et al. (2007). "2007 Guideline for the management of ureteral calculi". The Journal of Urology 178 (6): 2418–34. doi:10.1016/j.eururo.2007.09.039. PMID 17993340. http://www.urosource.com/fileadmin/European_Urology/Guidelines/Preminger_Guidelines.pdf.
- ^ a b Evan AP and McAteer JA : Q-effects of shock wave lithotripsy. In: Kidney Stones: Medical and Surgical Management. Edited by FL Coe, MJ Favus, CYC Pak, JH Parks and GM Preminger. Philadelphia:Lippincott-Raven 1996; Chapter 23, (pp. 549-560)
- ^ a b c Evan AP, and Willis LR (2007). Extracorporeal shock wave lithotripsy: complications. In: Smith's Textbook on Endourology. (Edited by AD Smith, GH Badlani, DH Bagley, RV Clayman, SG Docimo. Hamilton, Ontario, Canada: B C Decker, Inc. Chapter 41. (pp.353-365)
- ^ Lam JS and Gupta M, Chapter 25: Ureteral Stents, pp. 465-83 in Stoller and Meng (2007)
- ^ Marks AJ, Qiu J, Milner TE, Chan KF and Teichman JMH, Chapter 26: Laser Lithotripsy Physics, pp. 301-10 in Rao, Preminger and Kavanagh (2011)
- ^ Lieske, JC; Segura, JW (2004). "Chapter 7: Evaluation and Medical Management of Kidney Stones". In Potts, JM. Essential Urology: A Guide to Clinical Practice (1st ed.). Totowa, New Jersey: Humana Press. pp. 117–52. ISBN 9781588291097. http://www.amazon.com/Essential-Urology-Clinical-Practice-Current/dp/158829109X#reader_158829109X.
- ^ Eknoyan, G (2004). "History of urolithiasis". Clinical Reviews in Bone and Mineral Metabolism 2 (3): 177–85. doi:10.1385/BMM:2:3:177. ISSN 1534-8644.
- ^ Aulus Cornelius Celsus (1831). "Book VII, Chapter XXVI: Of the operation necessary in a suppression of urine, and lithotomy". In Collier, GF. A translation of the eight books of Aul. Corn. Celsus on medicine (2nd ed.). London: Simpkin and Marshall. pp. 306–14. http://books.google.com/books?id=p2kFAAAAQAAJ&pg=PA311#v=onepage&q&f=false.
- ^ a b Shah, J; Whitfield, HN (2002). "Urolithiasis through the ages". British Journal of Urology International 89 (8): 801–10. doi:10.1046/j.1464-410X.2002.02769.x. PMID 11972501.
- ^ a b Ellis, H (1969). A History of Bladder Stone. Oxford, England: Blackwell Scientific Publications. ISBN 9780632061402.
- ^ Bigelow, HJ (1878). Litholapaxy or rapid lithotrity with evacuation. Boston: A. Williams and Company. p. 29. http://books.google.com/?id=UUkSAAAAYAAJ&printsec=frontcover#v=onepage&q&f=false.
- ^ Coe, FL; Evan, A; Worcester, E (2008). "Chapter 114: Kidney stone disease". In Marks, AR; Neill, US. Science in medicine: the JCI textbook of molecular medicine. Part II: Kidney and urinary tract (1st ed.). Sudbury, Massachusetts: Jones and Bartlett Publishers. pp. 898–908. ISBN 9780763750831. http://books.google.com/books?vid=ISBN0763750832.
References
- Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine of the National Academies (2011). Ross, AC; Taylor, CL; Yaktine, AL et al.. eds. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press. ISBN 978-0-309-16394-1. http://www.nap.edu/catalog.php?record_id=13050.
- Rao, PN; Preminger, GM; Kavanagh, JP, eds (2011). Urinary Tract Stone Disease (1st ed.). London: Springer-Verlag. doi:10.1007/978-1-84800-362-0_26. ISBN 9781848003613. http://books.google.com/?id=LlJy5XJOkSkC&pg=PA302&lpg=PA302&dq=%22laser+has+become+the+dominant+laser+currently+used%22#v=onepage&q=%22laser%20has%20become%20the%20dominant%20laser%20currently%20used%22&f=false.
- Reilly Jr., RF; Perazella, MA, eds (2005). Nephrology in 30 Days (1st ed.). New York: The McGraw-Hill Companies, Inc.. ISBN 0071437010. http://books.google.com/?id=tBGhK_1L6rAC&pg=PA195&lpg=PA195&dq=%22Nephrology+in+30+Days%22+%22the+more+distal+in+the+ureter+it+is+located%22#v=onepage&q&f=false.
- Stoller, ML; Meng, MV, eds (2007). Urinary stone disease: the practical guide to medical and surgical management (1st ed.). Totowa, New Jersey: Humana Press. ISBN 9781592599721. http://books.google.com/?id=90CO-NgKrj4C&pg=PA480&lpg=PA480&dq=%22the+majority+of+indwelling+ureteral+stents+can+be+removed%22#v=onepage&q=%22the%20majority%20of%20indwelling%20ureteral%20stents%20can%20be%20removed%22&f=false.
External links
- National Digestive Diseases Information Clearinghouse, an information dissemination service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health, United States Public Health Service, United States Department of Health and Human Services
- National Endocrine and Metabolic Diseases Information Service, an information dissemination service of the NIDDK
- National Kidney and Urologic Diseases Information Clearinghouse, an information dissemination service of the NIDDK
- Kidney Stone Photographs
- kidney stones symptoms
- University of Pittsburgh Medical Center Low-Oxalate Diet (PDF)
- University of Pittsburgh Medical Center Low-Purine Diet(PDF)
Urinary system · Pathology · Urologic disease / Uropathy (N00–N39, 580–599) Abdominal .3 Mesangial proliferative · .4 Endocapillary proliferative .5/.6 Membranoproliferative/mesangiocapillaryBy conditionTubulopathy/
tubulitisAny/allAny/allGeneral syndromesOtherUreterPelvic UrethraUrethritis (Non-gonococcal urethritis) · Urethral syndrome · Urethral stricture/Meatal stenosis · Urethral caruncleAny/all Obstructive uropathy · Urinary tract infection · Retroperitoneal fibrosis · Urolithiasis (Bladder stone, Kidney stone, Renal colic) · Malacoplakia · Urinary incontinence (Stress, Urge, Overflow)Categories:- Kidney diseases
- Urological conditions
Wikimedia Foundation. 2010.