- Crystallization
-
"Crystallizing" and "Crystallized" redirect here. For the action-adventure video game, see Crystalis. For the 2009 song by The xx, see Crystalised.
Crystallization 
Concepts Crystallization · Crystal growth
Fractional crystallization
Recrystallization · Seed crystal
Single crystalFundamentals Nucleation · Crystal
Crystal structure · Solidv · crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. In chemical engineering crystallization occurs in a crystallizer. Crystallization is therefore an aspect of precipitation, obtained through a variation of the solubility conditions of the solute in the solvent, as compared to precipitation due to chemical reaction. Contents
Process
Time-lapse of growth of a citric acid crystal. The video covers an area of 2.0 by 1.5 mm and was captured over 7.2 min.
- See also crystallization dynamics section.
The crystallization process consists of two major events, nucleation and crystal growth. Nucleation is the step where the solute molecules dispersed in the solvent start to gather into clusters, on the nanometer scale (elevating solute concentration in a small region), that become stable under the current operating conditions. These stable clusters constitute the nuclei. However, when the clusters are not stable, they redissolve. Therefore, the clusters need to reach a critical size in order to become stable nuclei. Such critical size is dictated by the operating conditions (temperature, supersaturation, etc.). It is at the stage of nucleation that the atoms arrange in a defined and periodic manner that defines the crystal structure — note that "crystal structure" is a special term that refers to the relative arrangement of the atoms, not the macroscopic properties of the crystal (size and shape), although those are a result of the internal crystal structure.
The crystal growth is the subsequent growth of the nuclei that succeed in achieving the critical cluster size. Nucleation and growth continue to occur simultaneously while the supersaturation exists. Supersaturation is the driving force of the crystallization, hence the rate of nucleation and growth is driven by the existing supersaturation in the solution. Depending upon the conditions, either nucleation or growth may be predominant over the other, and as a result, crystals with different sizes and shapes are obtained (control of crystal size and shape constitutes one of the main challenges in industrial manufacturing, such as for pharmaceuticals). Once the supersaturation is exhausted, the solid–liquid system reaches equilibrium and the crystallization is complete, unless the operating conditions are modified from equilibrium so as to supersaturate the solution again.
Many compounds have the ability to crystallize with different crystal structures, a phenomenon called polymorphism. Each polymorph is in fact a different thermodynamic solid state and crystal polymorphs of the same compound exhibit different physical properties, such as dissolution rate, shape (angles between facets and facet growth rates), melting point, etc. For this reason, polymorphism is of major importance in industrial manufacture of crystalline products.
Crystallization in nature
 Snowflakes are a very well known example, where subtle differences in crystal growth conditions result in different geometries.
Snowflakes are a very well known example, where subtle differences in crystal growth conditions result in different geometries.
There are many examples of natural process that involve crystallization.
Geological time scale process examples include:
- Natural (mineral) crystal formation (see also gemstone);
- Stalactite/stalagmite, rings formation.
Usual time scale process examples include:
- Snow flakes formation (see also Koch snowflake);
- Honey crystallization (nearly all types of honey crystallize).
Artificial methods
For crystallization (see also recrystallization) to occur from a solution it must be supersaturated. This means that the solution has to contain more solute entities (molecules or ions) dissolved than it would contain under the equilibrium (saturated solution). This can be achieved by various methods, with (1) solution cooling, (2) addition of a second solvent to reduce the solubility of the solute (technique known as antisolvent or drown-out), (3) chemical reaction and (4) change in pH being the most common methods used in industrial practice. Other methods, such as solvent evaporation, can also be used. The spherical crystallization has some advantages (flowability and bioavailability) for the formulation of pharmaceutical drugs (see ref Nocent & al., 2001)
Applications
There are two major groups of applications for the artificial crystallization process: crystal production and purification.
Crystal production
From a material industry perspective:
- Macroscopic crystal production: for supply the demand of natural-like crystals with methods that "accelerate time-scale" for massive production and/or perfection.
- Tiny size crystals:
- Powder, sand and smaller sizes: using methods for powder and controlled (nanotechnology fruits) forms.
- Mass-production: on chemical industry, like salt-powder production.
- Sample production: small production of tiny crystals for material characterization. Controlled recrystallization is an important method to supply unusual crystals, that are needed to reveal the molecular structure and nuclear forces inside a typical molecule of a crystal. Many techniques, like X-ray crystallography and NMR spectroscopy, are widely used in chemistry and biochemistry to determine the structures of an immense variety of molecules, including inorganic compounds and bio-macromolecules.
- Thin film production.
- Powder, sand and smaller sizes: using methods for powder and controlled (nanotechnology fruits) forms.
Massive production examples:
- "Powder salt for food" industry;
- Silicon crystal wafer production.
- Production of sucrose from sugar beet, where the sucrose is crystallized out from an aqueous solution.
Purification
See also: Recrystallization (chemistry)Used to improve (obtaining very pure substance) and/or verify their purity.
Crystallization separates a product from a liquid feedstream, often in extremely pure form, by cooling the feedstream or adding precipitants which lower the solubility of the desired product so that it forms crystals.
Well formed crystals are expected to be pure because each molecule or ion must fit perfectly into the lattice as it leaves the solution. Impurities would normally not fit as well in the lattice, and thus remain in solution preferentially. Hence, molecular recognition is the principle of purification in crystallization. However, there are instances when impurities incorporate into the lattice, hence, decreasing the level of purity of the final crystal product. Also, in some cases, the solvent may incorporate into the lattice forming a solvate. In addition, the solvent may be 'trapped' (in liquid state) within the crystal formed, and this phenomenon is known as inclusion.
History
Crystallization is one of the pristine unit processes. It may be assumed that our ancestors used sodium chloride found in crevices of the surface rocks after drying caused by the sun: this process is still in use in modern solar ponds.
Other crystallization processes, for example sucrose production (this is the crystalline product with the largest world production, followed by sodium chloride), or in pigment manufacturing, were used in ancient times. These substances were sometimes produced by crystallizing the solutes of some more or less natural brine.
In more recent times, the fast expansion of the chemical industry has required a thorough study of the dynamics of crystallization, and this unit operation is now used in many industrial manufacturing areas: table salt, sugar, sodium sulfate, urea, just to name a few, are produced by crystallization from solutions.
Crystallizer technology has progressed alongside with the new processes. Once simple tanks in which, through cooling, evaporation or maybe through pH variation a crystal was obtained, nowadays continuous machines ensure a remarkable consistency in the product characteristics. Among the first models of modern crystallizers were probably the calandria type, being today the standard crystallizer for sucrose, and the Oslo, named after the Norwegian capital, since it was developed to produce salt in a climate not particularly fit for solar ponds, salt being widely used in Norway in stockfish production. The Oslo type was probably the first crystallizer designed specifically for the control of crystal growth.
Typical equipments
Equipments for the main industrial processes for crystallization.
- Tank crystallizers. Tank crystallization is an old method still used in some specialized cases. Saturated solutions, in tank crystallization, are allowed to cool in open tanks. After a period of time the mother liquid is drained and the crystals removed. Nucleation and size of crystals are difficult to control. Typically, labor costs are very high.
- Scraped surface crystallizers. One type of scraped surface crystallizer is the Swenson-Walker crystallizer, which consists of an open trough 0.6 m wide with a semicircular bottom having a cooling jacket outside. A slow-speed spiral agitator rotates and suspends the growing crystals on turning. The blades pass close to the wall and break off any deposits of crystals on the cooled wall. The product generally has a somewhat wide crystal-size distribution.
- Double-pipe scraped surface crystallizer. Also called a votator, this type of crystallizer is used in crystallizing ice cream and plasticizing margarine. Cooling water passes in the annular space. An internal agitator is fitted with spring-loaded scrapers that wipe the wall and provide good heat-transfer coefficients.
- Circulating-liquid evaporator-crystallizer. Also called Oslo crystallizer. Here supersaturation is reached by evaporation. The circulating liquid is drawn by the screw pump down inside the tube side of the condensing stream heater. The heated liquid then flows into the vapor space, where flash evaporation occurs, giving some supersaturation.The vapor leaving is condensed. The supersaturated liquid flows down the downflow tube and then up through the bed of fluidized and agitated crystals, which are growing in size. The leaving saturated liquid then goes back as a recycle stream to the heater, where it is joined by the entering fluid. The larger crystals settle out and slurry of crystals and mother liquid is withdrawn as a product.
- Circulating-magma vacuum crystallizer. The magma or suspension of crystals is circulated out of the main body through a circulating pipe by a screw pump. The magma flows though a heater, where its temperature is raised 2–6 K. The heated liquor then mixes with body slurry and boiling occurs at the liquid surface. This causes supersaturation in the swirling liquid near the surface, which deposits in the swirling suspended crystals until they leave again via the circulating pipe. The vapors leave through the top. A steam-jet ejector provides vacuum.
- Continuous oscillatory baffled crystallizer (COBC). The COBC is a tubular baffled crystallizer that offers plug flow under laminar flow conditions (low flow rates) with superior heat transfer coefficient, allowing controlled cooling profiles, e.g. linear, parabolic, discontinued, step-wise or any type, to be achieved. This gives much better control over crystal size, morphology and consistent crystal products.
Thermodynamic view
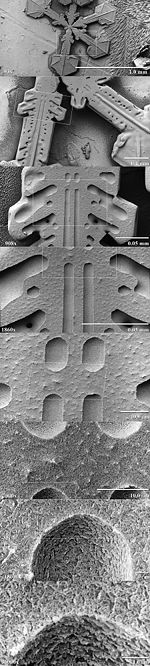 Low-temperature SEM magnification series for a snow crystal. The crystals are captured, stored, and sputter coated with platinum at cryo-temperatures for imaging.
Low-temperature SEM magnification series for a snow crystal. The crystals are captured, stored, and sputter coated with platinum at cryo-temperatures for imaging.
The nature of a crystallization process is governed by both thermodynamic and kinetic factors, which can make it highly variable and difficult to control. Factors such as impurity level, mixing regime, vessel design, and cooling profile can have a major impact on the size, number, and shape of crystals produced.
Now put yourself in the place of a molecule within a pure and perfect crystal, being heated by an external source. At some sharply defined temperature, a bell rings, you must leave your neighbours, and the complicated architecture of the crystal collapses to that of a liquid. Textbook thermodynamics says that melting occurs because the entropy, S, gain in your system by spatial randomization of the molecules has overcome the enthalpy, H, loss due to breaking the crystal packing forces:
T(Sliquid − Ssolid) > Hliquid − Hsolid
Gliquid < Gsolid
This rule suffers no exceptions when the temperature is rising. By the same token, on cooling the melt, at the very same temperature the bell should ring again, and molecules should click back into the very same crystalline form. The entropy decrease due to the ordering of molecules within the system is overcompensated by the thermal randomization of the surroundings, due to the release of the heat of fusion; the entropy of the universe increases.
But liquids that behave in this way on cooling are the exception rather than the rule; in spite of the second principle of thermodynamics, crystallization usually occurs at lower temperatures (supercooling). This can only mean that a crystal is more easily destroyed than it is formed. Similarly, it is usually much easier to dissolve a perfect crystal in a solvent than to grow again a good crystal from the resulting solution. The nucleation and growth of a crystal are under kinetic, rather than thermodynamic, control.
Crystallization dynamics
As mentioned above, a crystal is formed following a well-defined pattern, or structure, dictated by forces acting at the molecular level. As a consequence, during its formation process the crystal is in an environment where the solute concentration reaches a certain critical value, before changing status. Solid formation, impossible below the solubility threshold at the given temperature and pressure conditions, may then take place at a concentration higher than the theoretical solubility level. The difference between the actual value of the solute concentration at the crystallization limit and the theoretical (static) solubility threshold is called supersaturation and is a fundamental factor in crystallization dynamics. Supersaturation is the driving force for both the initial nucleation step and the following crystal growth, both of which could not occur in saturated or undersaturated conditions.
Nucleation
Nucleation is the initiation of a phase change in a small region, such as the formation of a solid crystal from a liquid solution. It is a consequence of rapid local fluctuations on a molecular scale in a homogeneous phase that is in a state of metastable equilibrium. Total nucleation is the sum effect of two categories of nucleation – primary and secondary.
Primary nucleation
Primary nucleation is the initial formation of a crystal where there are no other crystals present or where, if there are crystals present in the system, they do not have any influence on the process. This can occur in two conditions. The first is homogeneous nucleation, which is nucleation that is not influenced in any way by solids. These solids include the walls of the crystallizer vessel and particles of any foreign substance. The second category, then, is heterogeneous nucleation. This occurs when solid particles of foreign substances cause an increase in the rate of nucleation that would otherwise not be seen without the existence of these foreign particles. Homogeneous nucleation rarely occurs in practice due to the high energy necessary to begin nucleation without a solid surface to catalyse the nucleation.
Primary nucleation (both homogeneous and heterogeneous) has been modelled with the following:[1]

- B is the number of nuclei formed per unit volume per unit time.
- N is the number of nuclei per unit volume.
- knp is a rate constant.
- c is the instantaneous solute concentration.
- c* is the solute concentration at saturation.
- (c–c*) is also known as supersaturation.
- n is an empirical exponent that can be as large as 10, but generally ranges between 3 and 4.
Secondary nucleation
Secondary nucleation is the formation of nuclei attributable to the influence of the existing microscopic crystals in the magma.[2] The first type of known secondary crystallization is attributable to fluid shear, the other due to collisions between already existing crystals with either a solid surface of the crystallizer or with other crystals themselves. Fluid shear nucleation occurs when liquid travels across a Crystal at a high speed, sweeping away nuclei that would otherwise be incorporated into a Crystal, causing the swept-away nuclei to become new crystals. Contact nucleation has been found to be the most effective and common method for nucleation. The benefits include the following [2]
- Low kinetic order and rate-proportional to supersaturation, allowing easy control without unstable operation.
- Occurs at low supersaturation, where growth rate is optimum for good quality.
- Low necessary energy at which crystals strike avoids the breaking of existing crystals into new crystals.
- The quantitative fundamentals have already been isolated and are being incorporated into practice.
The following model, although somewhat simplified, is often used to model secondary nucleation:[1]

- k1 is a rate constant.
- MT is the suspension density.
- j is an empirical exponent that can range up to 1.5, but is generally 1.
- b is an empirical exponent that can range up to 5, but is generally 2.
Crystal growth
Crystal growth
Once the first small crystal, the nucleus, forms it acts as a convergence point (if unstable due to supersaturation) for molecules of solute touching – or adjacent to – the crystal so that it increases its own dimension in successive layers. The pattern of growth resembles the rings of an onion, as shown in the picture, where each colour indicates the same mass of solute; this mass creates increasingly thin layers due to the increasing surface area of the growing crystal. The supersaturated solute mass the original nucleus may capture in a time unit is called the growth rate expressed in kg/(m2*h), and is a constant specific to the process. Growth rate is influenced by several physical factors, such as surface tension of solution, pressure, temperature, relative crystal velocity in the solution, Reynolds number, and so forth.
The main values to control are therefore:
- Supersaturation value, as an index of the quantity of solute available for the growth of the crystal;
- Total crystal surface in unit fluid mass, as an index of the capability of the solute to fix onto the crystal;
- Retention time, as an index of the probability of a molecule of solute to come into contact with an existing crystal;
- Flow pattern, again as an index of the probability of a molecule of solute to come into contact with an existing crystal (higher in laminar flow, lower in turbulent flow, but the reverse applies to the probability of contact).
The first value is a consequence of the physical characteristics of the solution, while the others define a difference between a well- and poorly designed crystallizer.
Crystal size distribution
The appearance and size range of a crystalline product is extremely important in crystallization. If further processing of the crystals is desired, large crystals with uniform size are important for washing, filtering, transportation, and storage. The importance lies in the fact that large crystals are easier to filter out of a solution than small crystals. Also, larger crystals have a smaller surface area to volume ratio, leading to a higher purity. This higher purity is due to less retention of mother liquor which contains impurities, and a smaller loss of yield when the crystals are washed to remove the mother liquor. The theoretical crystal size distribution can be estimated as a function of operating conditions with a fairly complicated mathematical process called population balance theory (using population balance equations).
Main crystallization processes
The main factors influencing solubility are, as we saw above:
- Concentration
- Temperature
So we may identify two main families of crystallization processes:
- Cooling crystallization
- Evaporative crystallization
This division is not really clear-cut, since hybrid systems exist, where cooling is performed through evaporation, thus obtaining at the same time a concentration of the solution.
A crystallization process often referred to in chemical engineering is the Fractional crystallization. This is not a different process, rather a special application of one (or both) of the above.
Cooling crystallization
Application
Most chemical compounds, dissolved in most solvents, show the so-called direct solubility that is, the solubility threshold increases with temperature.
So, whenever the conditions are favourable, crystal formation results from simply cooling the solution. Here cooling is a relative term: austenite crystals in a steel form well above 1000 °C. An example of this crystallization process is the production of Glauber's salt, a crystalline form of sodium sulphate. In the picture, where equilibrium temperature is on the x-axis and equilibrium concentration (as mass percent of solute in saturated solution) in y-axis, it is clear that sulphate solubility quickly decreases below 32.5 °C. Assuming a saturated solution at 30 °C, by cooling it to 0 °C (note that this is possible thanks to the freezing-point depression), the precipitation of a mass of sulphate occurs corresponding to the change in solubility from 29% (equilibrium value at 30 °C) to approximately 4.5% (at 0 °C) – actually a larger crystal mass is precipitated, since sulphate entrains hydration water, and this has the side effect of increasing the final concentration.
There are of course limitation in the use of cooling crystallization:
- Many solutes precipitate in hydrate form at low temperatures: in the previous example this is acceptable, and even useful, but it may be detrimental when, for example, the mass of water of hydration to reach a stable hydrate crystallization form is more than the available water: a single block of hydrate solute will be formed – this occurs in the case of calcium chloride);
- Maximum supersaturation will take place in the coldest points. These may be the heat exchanger tubes which are sensitive to scaling, and heat exchange may be greatly reduced or discontinued;
- A decrease in temperature usually implies an increase of the viscosity of a solution. Too high a viscosity may give hydraulic problems, and the laminar flow thus created may affect the crystallization dynamics.
- It is of course not applicable to compounds having reverse solubility, a term to indicate that solubility increases with temperature decrease (an example occurs with sodium sulphate where solubility is reversed above 32.5 °C).
Cooling crystallizers
The simplest cooling crystallizers are tanks provided with a mixer for internal circulation, where temperature decrease is obtained by heat exchange with an intermediate fluid circulating in a jacket. These simple machines are used in batch processes, as in processing of pharmaceuticals and are prone to scaling. Batch processes normally provide a relatively variable quality of product along the batch.
The Swenson-Walker crystallizer is a model, specifically conceived by Swenson Co. around 1920, having a semicylindric horizontal hollow trough in which a hollow screw conveyor or some hollow discs, in which a refrigerating fluid is circulated, plunge during rotation on a longitudinal axis. The refrigerating fluid is sometimes also circulated in a jacket around the trough. Crystals precipitate on the cold surfaces of the screw/discs, from which they are removed by scrapers and settle on the bottom of the trough. The screw, if provided, pushes the slurry towards a discharge port.
A common practice is to cool the solutions by flash evaporation: when a liquid at a given T0 temperature is transferred in a chamber at a pressure P1 such that the liquid saturation temperature T1 at P1 is lower than T0, the liquid will release heat according to the temperature difference and a quantity of solvent, whose total latent heat of vaporization equals the difference in enthalpy. In simple words, the liquid is cooled by evaporating a part of it.
Evaporative crystallization
Another option is to obtain, at an approximately constant temperature, the precipitation of the crystals by increasing the solute concentration above the solubility threshold. To obtain this, the solute/solvent mass ratio is increased using the technique of evaporation. This process is of course insensitive to change in temperature (as long as hydration state remains unchanged).
All considerations on control of crystallization parameters are the same as for the cooling models.
Evaporative crystallizers
Most industrial crystallizers are of the evaporative type, such as the very large sodium chloride and sucrose units, whose production accounts for more than 50% of the total world production of crystals. The most common type is the forced circulation (FC) model (see evaporator). A pumping device (a pump or an axial flow mixer) keeps the crystal slurry in homogeneous suspension throughout the tank, including the exchange surfaces; by controlling pump flow, control of the contact time of the crystal mass with the supersaturated solution is achieved, together with reasonable velocities at the exchange surfaces. The Oslo, mentioned above, is a refining of the evaporative forced circulation crystallizer, now equipped with a large crystals settling zone to increase the retention time (usually low in the FC) and to roughly separate heavy slurry zones from clear liquid.
The DTB crystallizer
 DTB Crystallizer
DTB CrystallizerWhichever the form of the crystallizer, to achieve an effective process control it is important to control the retention time and the crystal mass, to obtain the optimum conditions in terms of crystal specific surface and the fastest possible growth. This is achieved by a separation – to put it simply – of the crystals from the liquid mass, in order to manage the two flows in a different way. The practical way is to perform a gravity settling to be able to extract (and possibly recycle separately) the (almost) clear liquid, while managing the mass flow around the crystallizer to obtain a precise slurry density elsewhere. A typical example is the DTB (Draft Tube and Baffle) crystallizer, an idea of Richard Chisum Bennett (a Swenson engineer and later President of Swenson) at the end of the 1950s. The DTB crystallizer (see images) has an internal circulator, typically an axial flow mixer – yellow – pushing upwards in a draft tube while outside the crystallizer there is a settling area in an annulus; in it the exhaust solution moves upwards at a very low velocity, so that large crystals settle – and return to the main circulation – while only the fines, below a given grain size are extracted and eventually destroyed by increasing or decreasing temperature, thus creating additional supersaturation. A quasi-perfect control of all parameters is achieved. This crystallizer, and the derivative models (Krystal, CSC, etc.) could be the ultimate solution if not for a major limitation in the evaporative capacity, due to the limited diameter of the vapour head and the relatively low external circulation not allowing large amounts of energy to be supplied to the system.
Gallery
- Solvent recrystallisation
- X-ray crystals
See also
- Micro-Pulling-Down
- Pumpable ice technology
- Quasicrystal
- Recrystallization (chemistry)
- Recrystallization (metallurgy)
- Seed crystal
- Single crystal
- Symplectite
- X-Ray Crystallography
References
- A. Mersmann, Crystallization Technology Handbook (2001) CRC; 2nd ed. ISBN 0-8247-0528-9
- Tine Arkenbout-de Vroome, Melt Crystallization Technology (1995) CRC ISBN 1-56676-181-6
- DTB Crystallizer
- Oslo Type Crystallizer
- "Small Molecule Crystallization" (PDF) at Illinois Institute of Technology website
- Glynn P.D. and Reardon E.J. (1990) "Solid-solution aqueous-solution equilibria: thermodynamic theory and representation". Amer. J. Sci. 290, 164–201.
- Geankoplis, C.J. (2003) "Transport Processes and Separation Process Principles". 4th Ed. Prentice-Hall Inc.
- Stanley SJ. (2006) Tomographic imaging during reactive precipitation: mixing with chemical reaction, Chemical Engineering Science, 61 (23), pp 7850–7863
- Nocent M, Bertocchi L, Espitalier F. & al. (2001) "Definition of a solvent system for spherical crystallization of salbutamol sulfate by quasi-emulsion solvent diffusion (QESD) method", Journal of Pharmaceutical Sciences, 90, 10, 1620–1627.
External links
v · d · eConcepts in asymmetric synthesis Chirality types Chirality · Stereocenter · Planar chirality · Chiral ligand · Axial chirality · Supramolecular chirality · Inherent chiralityChiral molecules Stereoisomer · Enantiomer · Diastereomer · Meso compound · Enantiomeric excess · Diastereomeric excess ·Analysis Optical rotation · Chiral derivatizing agents · NMR spectroscopy of stereoisomers · Ultraviolet-visible spectroscopy of stereoisomersChiral resolution Recrystallization · Kinetic resolution · Chiral column chromatography · Diastereomeric recrystallizationReactions v · Separation processes Processes Absorption · Acid-base extraction · Adsorption · Chromatography · Cross-flow filtration · Crystallization · Cyclonic separation · Dialysis (biochemistry) · Dissolved air flotation · Distillation · Drying · Electrochromatography · Electrofiltration · Filtration · Flocculation · Froth flotation · Gravity separation · Leaching (chemical science) · Liquid-liquid extraction · Microfiltration · Osmosis · Precipitation (chemistry) · Recrystallization · Reverse osmosis · Sedimentation · Solid Phase Extraction · Sublimation · Ultrafiltration (industrial)
Devices Multiphase systems Concepts Categories:- Inorganic chemistry
- Chemical engineering
- Chemical processes
- Separation processes
- Crystallography
- Geological processes
- Laboratory techniques
- Mineralogy
- Phases of matter
- Phase changes
- Phase transitions
Wikimedia Foundation. 2010.
Look at other dictionaries:
Crystallization — Crys tal*li*za tion (kr[i^]s tal*l[i^]*z[=a] sh[u^]n), n. [Cf. F. cristallization.] 1. (Chem. & Min.) The act or process by which a substance in solidifying assumes the form and structure of a crystal, or becomes crystallized; the formation of… … The Collaborative International Dictionary of English
Crystallization — Crystallization. См. Кристаллизация. (Источник: «Металлы и сплавы. Справочник.» Под редакцией Ю.П. Солнцева; НПО Профессионал , НПО Мир и семья ; Санкт Петербург, 2003 г.) … Словарь металлургических терминов
crystallization — index congealment Burton s Legal Thesaurus. William C. Burton. 2006 … Law dictionary
crystallization — That point in time where a contract or agreement triggers certain clauses in that contract. For example, when a bank appoints an Agent pursuant to its General Security Agreement, all the assets of the company in question, that are not secured by… … Glossary of Bankruptcy
crystallization — 1660s, noun of action from CRYSTALLIZE (Cf. crystallize) + ATION (Cf. ation). Figurative use is attested from 1842 … Etymology dictionary
crystallization — (Amer.) crys·tal·li·za·tion || ‚krɪstÉ™laɪ zeɪʃn n. act of crystallizing (taking form or shape of crystals), formation of crystals; forming into crystals; causing to take form or shape, causing to form into crystals (also… … English contemporary dictionary
crystallization — Becoming definite, clear, or unambiguous. The term crystallization is often used to refer to the triggering of an *asset or liability by the occurrence of an event … Auditor's dictionary
crystallization — kristalizacija statusas T sritis chemija apibrėžtis Medžiagos kristalų išsiskyrimas iš tirpalo, lydalo arba dujinės fazės. atitikmenys: angl. crystallization rus. кристаллизация … Chemijos terminų aiškinamasis žodynas
crystallization — kristalizacija statusas T sritis fizika atitikmenys: angl. crystallization vok. Kristallisation, f rus. кристаллизация, f pranc. cristallisation, f … Fizikos terminų žodynas
crystallization — kristalizacija statusas T sritis ekologija ir aplinkotyra apibrėžtis Medžiagos kristalų išsiskyrimas iš tirpalo, lydalo arba dujinės fazės. atitikmenys: angl. crystallization; crystallize vok. Kristallisation, f rus. кристализация, f … Ekologijos terminų aiškinamasis žodynas
Share the article and excerpts
Direct link
https://en-academic.com/dic.nsf/enwiki/633696 Do a right-click on the link above
and select “Copy Link”
Crystallization
- Crystallization
-
"Crystallizing" and "Crystallized" redirect here. For the action-adventure video game, see Crystalis. For the 2009 song by The xx, see Crystalised.
Crystallization 
Concepts Crystallization · Crystal growth
Fractional crystallization
Recrystallization · Seed crystal
Single crystalFundamentals Nucleation · Crystal
Crystal structure · Solid





