- Diastereomer
-
Diastereomers 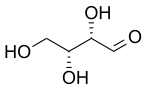
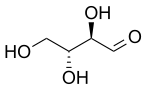
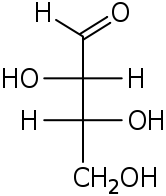
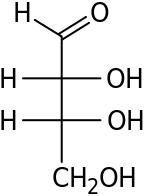
D-Threose D-Erythrose - Erythro redirects here. For the fictional planet, see Erythro (Asimov).
Diastereomers (sometimes called diastereoisomers) are stereoisomers that are not enantiomers.[1] Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other.[2] When two diastereoisomers differ from each other at only one stereocenter they are epimers. Each stereocenter gives rise to two different configurations and thus increases the number of stereoisomers by a factor of two.
Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another.[3] Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their mirror image. Diastereomers have different physical properties and different reactivity, unlike enantiomers.
Cis-trans isomerism and conformational isomerism are also forms of diastereomerism.
Diastereoselectivity is the preference for the formation of one or more than one diastereomer over the other in an organic reaction.
Contents
Erythro / threo
Two common prefixes used to distinguish diastereomers are threo and erythro (which correspond to the more intuitive anti and syn labels, respectively). When drawn in the Fischer projection the erythro isomer has two identical substituents on the same side and the threo isomer has them on opposite sides.[4] When drawn as a zig-zag chain, the erythro isomer has two identical substituents on different sides of the plane (anti). The names are derived from the diastereomeric aldoses erythrose (a syrup) and threose (melting point 126 °C).
Another threo compound is threonine, one of the essential amino acids. The erythro diastereomer is called allo-threonine.
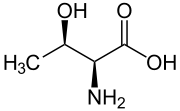
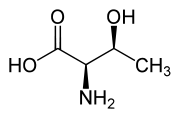
L-Threonine (2S,3R) and D-Threonine (2R,3S) 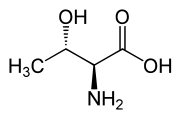
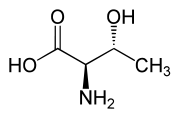
L-allo-Threonine (2S,3S) and D-allo-Threonine (2R,3R) Multiple stereocenters
If a molecule contains two asymmetric carbons, there are up to 4 possible configurations, and they cannot all be non-superimposable mirror images of each other. The possibilities continue to multiply as there are more asymmetric centers in a molecule. In general, the number of configurational isomers of a molecule can be determined by calculating 2n, where n = the number of chiral centers in the molecule. This holds true except in cases where the molecule has meso forms.
Example
Tartaric acid contains two asymmetric centers, but two of the "isomers" are equivalent and together are called a meso compound. This configuration is not optically active, while the remaining two isomers are D- and L- mirror images, i.e., enantiomers. The meso form is a diastereomer of the other forms.


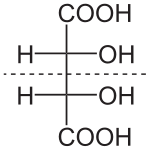
(natural) tartaric acid
L-(+)-tartaric acid
dextrotartaric acidD-(-)-tartaric acid
levotartaric acidmesotartaric acid
(1:1)
DL-tartaric acid
"racemic acid"The families of 4, 5 and 6 carbon carbohydrates contain many diastereomers because of the large numbers of asymmetric centres in these molecules.
Applications
As stated, two enantiomers will have identical chemical properties, while diastereomers will not. This knowledge is harnessed in chiral synthesis to separate a mixture of enantiomers. This is the principle behind chiral resolution. After preparing the diastereomers, they are separated by chromatography or recrystallization.
Therefore, for two diastereomers, two peaks are observed when analysed using NMR.
See also
Cahn-Ingold-Prelog priority rules for nomenclature.
References
- ^ IUPAC "Gold Book" diastereoisomerism doi:10.1351/goldbook.D01679
- ^ Garrett, R.H.; Grisham, C.M. (2005), Biochemistry 3rd ed., Belmont CA: Thomson, p. 205, ISBN 0534410200.
- ^ IUPAC "Gold Book" enantiomer doi:10.1351/goldbook.E02069
- ^ Modern physical organic chemistry Eric V. Anslyn,Dennis A. Dougherty 2006
Concepts in asymmetric synthesis Chirality types Chirality · Stereocenter · Planar chirality · Chiral ligand · Axial chirality · Supramolecular chirality · Inherent chiralityChiral molecules Stereoisomer · Enantiomer · Diastereomer · Meso compound · Enantiomeric excess · Diastereomeric excess ·Analysis Optical rotation · Chiral derivatizing agents · NMR spectroscopy of stereoisomers · Ultraviolet-visible spectroscopy of stereoisomersChiral resolution Recrystallization · Kinetic resolution · Chiral column chromatography · Diastereomeric recrystallizationReactions Categories:- Stereochemistry
- Isomerism
Wikimedia Foundation. 2010.
