- Enantiomer
-
This article is about the concept in chemistry. For a discussion of enantiomers in mathematics, see Chirality (mathematics).
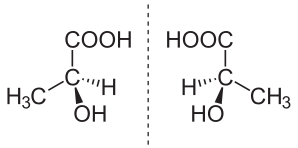 (S)-(+)-lactic acid (left) and (R)-(–)-lactic acid (right) are nonsuperposable mirror images of each other
(S)-(+)-lactic acid (left) and (R)-(–)-lactic acid (right) are nonsuperposable mirror images of each otherIn chemistry, an enantiomer (
 /ɨˈnæntɪ.ɵmər/ ə-nan-tee-ə-mər; from the Greek ἐνάντιος, opposite, and μέρος, part or portion) is one of two stereoisomers that are mirror images of each other that are non-superposable (not identical), much as one's left and right hands are the same except for opposite orientation.[1] It can be clearly understood if you try to place your hands one over the other without touching the back or palm of the left to the same of the right. You observe that the thumb of one is always over the little-finger of the other, thus explaining the non-superimposable or non-coincident property known as chirality.
/ɨˈnæntɪ.ɵmər/ ə-nan-tee-ə-mər; from the Greek ἐνάντιος, opposite, and μέρος, part or portion) is one of two stereoisomers that are mirror images of each other that are non-superposable (not identical), much as one's left and right hands are the same except for opposite orientation.[1] It can be clearly understood if you try to place your hands one over the other without touching the back or palm of the left to the same of the right. You observe that the thumb of one is always over the little-finger of the other, thus explaining the non-superimposable or non-coincident property known as chirality.Organic compounds that contain an asymmetric (chiral) Carbon usually have two non-superimposable structures. These two structures are mirror images of each other and are, thus, commonly called enantiomorphs (enantio = opposite ; morph = form) Hence, optical isomerism (which occurs due to these same mirror-image properties) is now commonly referred to as enantiomerism
Enantiopure compounds refer to samples having, within the limits of detection, molecules of only one chirality.[2]
Enantiomers have, when present in a symmetric environment, identical chemical and physical properties except for their ability to rotate plane-polarized light (+/−) by equal amounts but in opposite directions (although the polarized light can be considered an asymmetric medium). A mixture of equal parts of an optically active isomer and its enantiomer is termed racemic and has zero (± 0) net rotation of plane-polarized light.
Enantiomers of each other often show different chemical reactions with other substances that are also enantiomers. Since many molecules in the body of living beings are enantiomers themselves, there is often a marked difference in the effects of two enantiomers on living beings. In drugs, for example, often only one of a drug's enantiomers is responsible for the desired physiologic effects, while the other enantiomer is less active, inactive, or sometimes even responsible for adverse effects (unwanted side-effects).
Owing to this discovery, drugs composed of only one enantiomer ("enantiopure") can be developed to enhance the pharmacological efficacy and sometimes do away with some side effects. An example of this kind of drug is eszopiclone (Lunesta), which is enantiopure and therefore is given in doses that are exactly 1/2 of the older, racemic mixture called zopiclone. In the case of eszopiclone, the S enantiomer is responsible for all the desired effects, though the other enantiomer seems to be inactive; while an individual must take 2 mg of zopiclone to get the same therapeutic benefit as they would receive from 1 mg of eszopiclone, that appears to be the only difference between the two drugs.
Contents
Naming conventions
Main article: Chirality (chemistry)#Naming conventionsCriterion of Enantiomerism
Most compounds that contain one or more asymmetric Carbon atoms show enantiomerism. But this is not always true.
There are a few known compounds that do have asymmetric Carbons but being non-dissymetric do not show enantiomerism. Thus, meso tartaric acid has two asymmetric Carbons but is still optically inactive. Likewise, trans-cyclohexane - 1,4 - dicarboxylic acid has asymmetric Carbons but also has a centre of symmetry (thereby making it a non-dissymmetric compound) and, therefore, exhibits no enantiomerism.
Examples
 Enantiomers of mecoprop (2-(4-chloro-2-methylphenoxy)propanoic acid)
Enantiomers of mecoprop (2-(4-chloro-2-methylphenoxy)propanoic acid)
An example of such an enantiomer is the sedative thalidomide. It was sold in a number of countries across the world from 1957 until 1961, when it was withdrawn from the market after being found to be a cause of birth defects.
In the herbicide mecoprop, the carboxyl group and the hydrogen atom on the central C-atom are exchanged (with the screen as plane of symmetry). After rotating one of the isomers 180 degrees (in the same plane), the two are still mirror images of each other. The mirror image of each enantiomer is superposable on the other enantiomer.
Another example is the antidepressant drugs escitalopram and citalopram. Citalopram is a racemate [1:1 mixture of (S)-citalopram and (R)-citalopram]; escitalopram [(S)-citalopram] is a pure enantiomer. The dosages for escitalopram are typically 1/2 of those for citalopram.
Enantioselective preparations
See also: chiral resolution and asymmetric synthesisThere are two main strategies for the preparation of enantiopure compounds. The first is known as chiral resolution. This method involves preparing the compound in racemic form, and separating it into its isomers. In his pioneering work, Louis Pasteur was able to isolate the isomers of tartaric acid because they crystallize from solution as crystals each with a different symmetry. A less common method is by enantiomer self-disproportionation.
The second strategy is asymmetric synthesis: the use of various techniques to prepare the desired compound in high enantiomeric excess. Techniques encompassed include the use of chiral starting materials (chiral pool synthesis), the use of chiral auxiliaries and chiral catalysts, and the application of asymmetric induction. The use of enzymes (biocatalysis) may also produce the desired compound.
Enantioconvergent synthesis is the synthesis of one enantiomer from a racemic precursor molecule utilizing both enantiomers. Thus, the two enantiomers of the reactant produce a single enantiomer of product.
Enantiopure medications
Main article: Enantiopure drugAdvances in industrial chemical processes have made it economical for pharmaceutical manufacturers to take drugs that were originally marketed as a racemic mixture and market the individual enantiomers. In some cases, the enantiomers have genuinely different effects. In other cases, there may be no clinical benefit to the patient. In some jurisdictions, single-enantiomer drugs are separately patentable from the racemic mixture.[3] It is possible that both enantiomers are active. Or, it may be that only one is active, in which case separating the mixture has no objective benefits, but extends the drug's patentability.[4]
Quasi-enantiomers
These molecular species are not strictly enantiomers, but they behave as if they are. Quasi-enantiomers have applications in parallel kinetic resolution (see kinetic resolution).[5]
See also
- Enantiopure drug
- Stereochemistry
- Dynamic stereochemistry
- Chirality (chemistry)
- Diastereomers
- Stereogenic
- Atropisomerism
- Antipode (chemistry)
External Links
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "enantiomer".
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "enantiomerically pure (enantiopure)".
- ^ http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2009/11/news_detail_000083.jsp&jsenabled=true
- ^ Merrill Goozner (2004) (excerpt). The $800 Million Pill: The Truth Behind the Cost of New Drugs. University of California Press. ISBN 0-520-23945-8. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1395782#id973953.
- ^ G.S. Coumbarides, M. Dingjan, J. Eames, A. Flinn, J. Northen and Y. Yohannes, Tetrahedron Lett. 46 (2005), p. 2897er ({{pro
Concepts in asymmetric synthesis Chirality types Chirality · Stereocenter · Planar chirality · Chiral ligand · Axial chirality · Supramolecular chirality · Inherent chiralityChiral molecules Stereoisomer · Enantiomer · Diastereomer · Meso compound · Enantiomeric excess · Diastereomeric excess ·Analysis Optical rotation · Chiral derivatizing agents · NMR spectroscopy of stereoisomers · Ultraviolet-visible spectroscopy of stereoisomersChiral resolution Recrystallization · Kinetic resolution · Chiral column chromatography · Diastereomeric recrystallizationReactions Categories:- Stereochemistry
- Isomerism
Wikimedia Foundation. 2010.

