- Chiral resolution
-
Chiral resolution in stereochemistry is a process for the separation of racemic compounds into their enantiomers.[1] It is an important tool in the production of optically active drugs. Other terms with the same meaning are optical resolution and mechanical resolution.
One disadvantage of chiral resolution of racemates compared to direct asymmetric synthesis of one of the enantiomers is that only 50% of a desired enantiomer is obtained. Several methods exist.
Contents
Resolution by crystallization
Typically 5-10% of all racemates are known to crystallize as mixtures of enantiopure crystals, so-called conglomerates [2]. Louis Pasteur was the first to conduct chiral resolution when he discovered the concept of optical activity in the first place by the manual separation of left-handed and right-handed tartaric acid crystals in 1849. Later in 1882 he went on to demonstrate that by seeding a supersaturated solution of sodium ammonium tartrate with a d-crystal on one side of the reactor and a l-crystal on the opposite side, crystals of opposite handedness will form on the opposite side of the reactor.
This type of resolution called spontaneous resolution has also been demonstrated with racemic methadone.[3] In a typical setup 50 grams dl-methadone is dissolved in petroleum ether and concentrated. Two millimeter-sized d- and l-crystals are added and after stirring for 125 hours at 40°C two large d- and l-crystals are recovered in 50% yield.
Another form of direct crystallization is preferential crystallization also called resolution by entrainment of one of the enantiomers. For example an added seed of (-)-hydrobenzoin to an ethanol solution of (±)-hydrobenzoin will have the (-)-enantiomer crystallizing out and after 15 cycles 97% optical purity can be obtained.
Chiral resolving agents
Derivatization of racemic compounds is possible with optically pure reagents forming pairs of diastereomers which can be separated by conventional techniques in physical chemistry. Derivatization is possible by salt formation between an amine and a carboxylic acid. Simple deprotonation affords the pure enantiomer. Examples of so-called chiral resolving agents are tartaric acid and brucine. The method was introduced (again) by Louis Pasteur in 1853 by resolving racemic tartaric acid with optically active (+)-cinchotoxine.
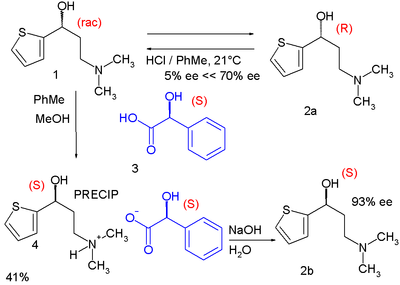
One modern-day method of chiral resolution is used in the organic synthesis of the drug Duloxetine:[4]
In one of its steps the racemic alcohol 1 is dissolved in a mixture of toluene and methanol to which solution is added optically active (S)-mandelic acid 3. The alcohol (S)-enantiomer forms an insoluble diastereomeric salt with the mandelic acid and can be filtered from the solution. Simple deprotonation with sodium hydroxide liberates free (S)-alcohol. In the meanwhile the (R)-alcohol remains in solution unaffected and is recycled back to the racemic mixture by epimerization with hydrochloric acid in toluene. This process is known as RRR synthesis in which the R's stand for Resolution-Racemization-Recycle.
Chiral column chromatography
- In chiral column chromatography the stationary phase is made chiral with similar resolving agents as described above.
References
- ^ William H. Porter (1991). "Resolution of chiral drugs". Pure Appl. Chem. 63 (8): 1119–1122. doi:10.1351/pac199163081119. http://www.iupac.org/publications/pac/1991/pdf/6308x1119.pdf.
- ^ Enantiomers, racemates, and resolutions Jean Jacques, André Collet, Samuel H Wilen 1981 ISBN 0-471-08058-6
- ^ Harold E. Zaugg (1955). "A Mechanical Resolution of dl-Methadone Base". J. Am. Chem. Soc. 77 (10): 2910. doi:10.1021/ja01615a084.
- ^ Yoshito Fujima, Masaya Ikunaka, Toru Inoue, and Jun Matsumoto (2006). "Synthesis of (S)-3-(N-Methylamino)-1-(2-thienyl)propan-1-ol: Revisiting Eli Lilly's Resolution-Racemization-Recycle Synthesis of Duloxetine for Its Robust Processes". Org. Process Res. Dev. 10 (5): 905–913. doi:10.1021/op06011.
Concepts in asymmetric synthesis Chirality types Chirality · Stereocenter · Planar chirality · Chiral ligand · Axial chirality · Supramolecular chirality · Inherent chiralityChiral molecules Stereoisomer · Enantiomer · Diastereomer · Meso compound · Enantiomeric excess · Diastereomeric excess ·Analysis Optical rotation · Chiral derivatizing agents · NMR spectroscopy of stereoisomers · Ultraviolet-visible spectroscopy of stereoisomersChiral resolution Recrystallization · Kinetic resolution · Chiral column chromatography · Diastereomeric recrystallizationReactions Categories:
Wikimedia Foundation. 2010.