- Mandelic acid
-
Mandelic acid[1] 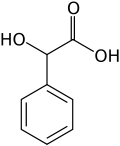 2-Hydroxy-2-phenylacetic acidOther namesMandelic acid
2-Hydroxy-2-phenylacetic acidOther namesMandelic acid
Phenylglycolic acidIdentifiers CAS number 90-64-2  , 611-71-2 (R), 17199-29-0 (S)
, 611-71-2 (R), 17199-29-0 (S)PubChem 1292 ChemSpider 1253 
UNII NH496X0UJX 
ChEBI CHEBI:35825 
ChEMBL CHEMBL1609 
Jmol-3D images Image 1 - O=C(O)C(O)c1ccccc1
Properties Molecular formula C8H8O3 Molar mass 152.15 g mol−1 Appearance White crystalline powder Density 1.30 g/cm3 Melting point 119 °C (optically pure: 132 – 135 °C)
Solubility in water 15.87 g per 100 mL Acidity (pKa) 3.41[2] Hazards Flash point 162.6 °C (324.7 °F) Related compounds Related compounds mandelonitrile, phenylacetic acid, vanillylmandelic acid  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Mandelic acid is an aromatic alpha hydroxy acid with the molecular formula C6H5CH(OH)CO2H. It is a white crystalline solid that is soluble in water and polar organic solvents. It is a useful precursor to various drugs. Since the molecule is chiral, it exists in either of two enantiomers as well as the racemic mixture, known as paramandelic acid.
Isolation and synthesis
Mandelic acid was discovered while heating amygdalin, an extract of bitter almonds, with diluted hydrochloric acid. The name is derived from the German "Mandel" for "almond". Derivatives of mandelic acid are formed as a result of metabolism of adrenaline and noradrenaline by monoamine oxidase and catechol-O-methyl transferase.
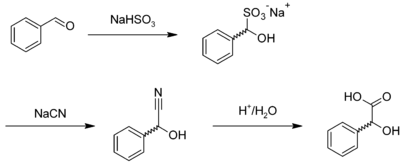
Mandelic acid is usually prepared by the acid-catalysed hydrolysis of mandelonitrile,[3] which is the cyanohydrin of benzaldehyde. Mandelonitrile can also be prepared by reacting benzaldehyde with sodium bisulfite to give the corresponding adduct, forming mandelonitrile with sodium cyanide, which is hydrolyzed:[4]
Alternatively, it arises by base hydrolysis of phenylchloroacetic acid and dibromacetophenone.[5] It also arises by heating phenylglyoxal with alkalis.
Uses
Mandelic acid has a long history of use in the medical community as an antibacterial, particularly in the treatment of urinary tract infections.[6] It has also been used as an oral antibiotic. In skin care, it is also an alternative to glycolic acid in skin care products. Mandelic acid is also advantageous in that it possesses antibacterial properties. Its use as a skincare modality was pioneered by Dr James E. Fulton, who developed vitamin A acid (tretinoin, Retin A) in 1969.[7] On the basis of this research, dermatologists now suggest mandelic acid for a wide variety of skin concerns, from acne to wrinkles; it is especially good in the treatment of adult acne as it addresses both of these concerns.[citation needed] Mandelic acid products have been used as an alternative treatment for rosacea sufferers, as it reduces inflammation and redness.[citation needed] Mandelic acid is also recommended for pre- and post-laser treatment, reducing the amount of redness and irritation caused by laser resurfacing.
The drugs cyclandelate and homatropine are esters of mandelic acid.
References
- ^ Merck Index, 11th Edition, 5599.
- ^ Bjerrum, J., et al. Stability Constants, Chemical Society, London, 1958.
- ^ Edwin Ritzer and Rudolf Sundermann “Hydroxycarboxylic Acids, Aromatic” in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a13_519
- ^ Corson, B. B.; Dodge, R. A.; Harris, S. A.; Yeaw, J. S. (1941), "Mandelic Acid", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1p0336; Coll. Vol. 1: 336
- ^ J. G. Aston, J. D. Newkirk, D. M. Jenkins, and Julian Dorsky (1952), "Mandelic Acid", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0538; Coll. Vol. 3: 538
- ^ Putten, P. L. (1979). "Mandelic acid and urinary tract infections". Antonie van Leeuwenhoek 45 (4): 622. doi:10.1007/BF00403669.
- ^ Taylor, MB. (1999). "Summary of mandelic acid for the improvement of skin conditions". Cosmetic Dermatology 21: 26–28.
 This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). Encyclopædia Britannica (11th ed.). Cambridge University Press.
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). Encyclopædia Britannica (11th ed.). Cambridge University Press.
Antibacterials: others (J01X) Other/ungrouped bornane: Xibornol
alpha hydroxy acid: Mandelic acid
nitroquinoline: Nitroxoline
pyran/fatty acid: Isoleucine-tRNA ligase inhibitors (Mupirocin)Categories:- Hydroxy acids
- Aromatic compounds
Wikimedia Foundation. 2010.