- Cyanohydrin
-
A cyanohydrin is a functional group found in organic compounds. Cyanohydrins have the formula R2C(OH)CN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids. Cyanohydrins can be formed by the cyanohydrin reaction, which involves treating a ketone or an aldehyde with hydrogen cyanide (HCN) in the presence of excess amounts of sodium cyanide (NaCN) as a catalyst:
- RR’C=O + HCN → RR’C(OH)CN
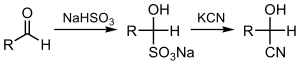
In this reaction, the nucleophilic CN− ion attacks the electrophilic carbonyl carbon in the ketone, followed by protonation by HCN, thereby regenerating the cyanide anion. Cyanohydrins are also prepared by displacement of sulfite by cyanide salts:[1]
Cyanohydrins are intermediates in the Strecker amino acid synthesis.
Contents
Acetone cyanohydrins
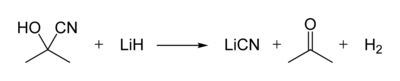
Acetone cyanohydrin, (CH3)2C(OH)CN is the cyanohydrin of acetone. It is generated as an intermediate in the industrial production of methyl methacrylate.[2] In the laboratory, this liquid serves as a source of HCN, which is inconveniently volatile.[3] Thus, acetone cyanohydrin can be used for the preparation other cyanohydrins, for of HCN to Michael acceptors, and for the formylation of arenes. Treatment of this cyanohydrin with lithium hydride affords anhydrous lithium cyanide:
Other cyanohydrins
Mandelonitrile, with the formula C6H5CH(OH)CN, occurs in small amounts in the pits of some fruits.[1] Related cyanogenic glycosides are known, such as amygdalin.
Glycolonitrile, also called hydroxyacetonitrile or formaldehyde cyanohydrin, is the organic compound with the formula HOCH2CN. It is the simplest cyanohydrin, being derived from formaldehyde.[4]
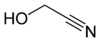
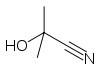
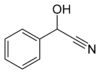
glycolonitrile acetone cyanohydrin mandelonitrile References
- ^ a b Corson, B. B.; Dodge, R. A.; Harris, S. A.; Yeaw, J. S. (1941), "Mandelic Acid", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv1p0336; Coll. Vol. 1: 336
- ^ William Bauer, Jr. "Methacrylic Acid and Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. DOI: 10.1002/14356007.a16_441. Article Online Posting Date: June 15, 2000
- ^ Haroutounian, S. A. ”Acetone Cyanohydrin” Encyclopedia of Reagents for Organic Synthesis 2001, John Wiley & Sons. DOI: 10.1002/047084289X.ra014
- ^ Gaudry, R. (1955), "Glycolonitrile", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0436; Coll. Vol. 3: 436
External links
- IUPACs Gold Book definition of cyanohydrins [1]
Categories:- Functional groups
- Cyanohydrins
Wikimedia Foundation. 2010.