- Amygdalin
-
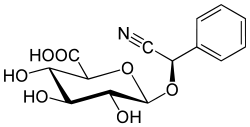
Amygdalin 
 [(6-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy](phenyl)acetonitrile
[(6-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy](phenyl)acetonitrileIdentifiers CAS number 29883-15-6 
PubChem 34751 ChemSpider 570897 
UNII 214UUQ9N0H 
MeSH Amygdalin ChEBI CHEBI:17019 
ChEMBL CHEMBL461727 
Jmol-3D images Image 1
Image 2- O[C@@H]3[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]3OC[C@H]2O[C@@H](OC(C#N)c1ccccc1)[C@H](O)[C@@H](O)[C@@H]2O
N#C[C@H](O[C@@H]2O[C@H](CO[C@@H]1O[C@@H]([C@@H](O)[C@H](O)[C@H]1O)CO)[C@@H](O)[C@H](O)[C@H]2O)c3ccccc3
- InChI=1S/C20H27NO11/c21-6-10(9-4-2-1-3-5-9)30-20-18(28)16(26)14(24)12(32-20)8-29-19-17(27)15(25)13(23)11(7-22)31-19/h1-5,10-20,22-28H,7-8H2/t10-,11+,12+,13+,14+,15-,16-,17+,18+,19+,20+/m0/s1

Key: XUCIJNAGGSZNQT-JHSLDZJXSA-N
InChI=1/C20H27NO11/c21-6-10(9-4-2-1-3-5-9)30-20-18(28)16(26)14(24)12(32-20)8-29-19-17(27)15(25)13(23)11(7-22)31-19/h1-5,10-20,22-28H,7-8H2/t10-,11+,12+,13+,14+,15-,16-,17+,18+,19+,20+/m0/s1
Key: XUCIJNAGGSZNQT-JHSLDZJXBT
Properties Molecular formula C20H27NO11 Molar mass 457.43 g mol−1 Related compounds Related compounds vicianin  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Amygdalin (from Greek: ἀμυγδάλη amygdálē “almond”), C20H27NO11, is a glycoside initially isolated from the seeds of the tree Prunus dulcis, also known as bitter almonds, by Pierre-Jean Robiquet and A. F. Boutron-Charlard in 1830,[1] and subsequently investigated by Liebig and Wöhler in 1830. Several other related species in the genus of Prunus, including apricot (Prunus armeniaca) and black cherry (Prunus serotina),[2] also contain amygdalin.
Since the early 1950s, a modified form of amygdalin has been promoted under the names laetrile and "Vitamin B17" as a cancer cure, but it is not a vitamin,[3] and studies have found it to be ineffective and potentially toxic[4][5][6] as a possible cause of cyanide poisoning.[7] The promotion of laetrile to treat cancer has been described in the scientific literature as a canonical example of quackery,[8][9][10] with Irving Lerner of the University of Minnesota describing it as "the slickest, most sophisticated, and certainly the most remunerative cancer quack promotion in medical history."[3]
Contents
Chemistry
Amygdalin is extracted from almond or apricot kernel cake by boiling in ethanol; on evaporation of the solution and the addition of diethyl ether, amygdalin is precipitated as white minute crystals. Liebig and Wöhler were already able to find three decomposition products of the newly discovered amygdalin: sugar, benzaldehyde, and prussic acid (hydrogen cyanide).[11] Later research showed that sulfuric acid decomposes it into D-glucose, benzaldehyde, and prussic acid; while hydrochloric acid gives mandelic acid, D-glucose, and ammonia.[12]
Several glucosidase enzymes are known to act on amygdalin, leading to its decomposition by various pathways. Maltase causes partial degradation, giving D-glucose and mandelic nitrile glucoside, C6H5CH(CN)O·C6H11O5. Emulsin, on the other hand, decomposes it into benzaldehyde, cyanide, and two molecules of glucose; this enzyme occurs in the bitter almond, and consequently the seeds invariably contain free cyanide and benzaldehyde. An "amorphous amygdalin" is said to occur in the cherry laurel (Prunus laurocerasus). Lastly, amygdalin beta-glucosidase and prunasin beta-glucosidase consecutively catalyze loss of the two glucose units to yield mandelonitrile, which can then decompose to form free cyanide and benzaldehyde.
Natural amygdalin has the R configuration at the chiral benzyl center. Under mild basic conditions, this stereogenic center epimerizes; the S epimer is called neoamygdalin.[13]
Laetrile
Amygdalin is sometimes confused with laevomandelonitrile, also called laetrile for short; however, amygdalin and laetrile are different chemical compounds.[13] Laetrile, which was patented in the United States, is a semi-synthetic molecule sharing part of the amygdalin structure, while the "laetrile" made in Mexico is usually amygdalin, the natural product obtained from crushed apricot pits, or neoamygdalin.[14]
Laetrile has a melting point of 214 to 216 degrees Celsius.[15] Its Chemical Abstracts Service Registry Number is 1332-94-1 and its International Union of Pure and Applied Chemistry nomenclature name is (2S,3S,4S,5R,6R)-6-[(R)-cyano(phenyl)methoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid. Laetrile's chemical formula is C14H15NO7 and its PubChem number is 5484354.[16] Its molecular weight is 309.2714 grams/mole.[16] Laetrile is also classified as a cyanogenic glycoside.[17]
Though it is sometimes sold as "Vitamin B17", it is not a vitamin. Amygdalin/laetrile was claimed to be a vitamin by chemist Ernst T. Krebs in the hope that if classified as a nutritional supplement it would escape the federal legislation regarding the marketing of drugs. He could also capitalise on the public fad for vitamins at that time.[3]
Toxicity
The metabolism of amygdalin produces hydrogen cyanide, a potent toxin. Beta-glucosidase, one of the enzymes that catalyzes the release of cyanide from amygdalin, is present in the human small intestine and in a variety of common foods. This leads to an unpredictable and potentially lethal toxicity when amygdalin or laetrile is taken orally.[3][18][19] Ingestion of purified amygdalin or apricot seeds can cause severe toxicity and death due to cyanide poisoning.[14] Numerous case reports in the medical literature describe serious cyanide poisoning in patients who ingested laetrile as a cancer treatment.[8][20] Blood cyanide concentrations may be measured as a means of confirming the diagnosis in hospitalized patients or to assist in the forensic investigation of a fatal overdose.[21]
Some laetrile promoters have claimed that the cyanide generated by laetrile is harmlessly detoxified by the mitochondrial enzyme rhodanese. However, these claims are false, as regardless of the level of rhodanese present, the body's available stores of cystine, cysteine, and other sulfur compounds necessary to detoxify cyanide are rapidly depleted in laetrile poisoning.[8]
Cancer treatment
Amygdalin was first isolated in 1830. In 1845 it was used as a cancer treatment in Russia, and in the 1920s in the United States, but it was considered too poisonous.[22] In the 1950s, a purportedly non-toxic, synthetic form was patented for use as a meat preservative,[23] and later marketed as laetrile for cancer treatment.[22]
The U.S. Food and Drug Administration prohibited the interstate shipment of amygdalin and laetrile in 1977.[24][25] Thereafter, 27 U.S. states legalized the use of amygdalin within those states.[26]
Initial studies at Sloan-Kettering
In 1972, Memorial Sloan-Kettering Cancer Center (MSKCC) board member Benno C. Schmidt, Sr. convinced the hospital to test laetrile so that he could assure others of its ineffectiveness "with some conviction."[27] Kanematsu Sugiura, the scientist who performed the requested tests, found that laetrile inhibited secondary tumors in mice, though it did not destroy the primary tumors. He repeated the experiment several times with the same results. However, three other researchers were unable to confirm Sugiura's results. While these uncontrolled and inconclusive results were considered too preliminary to publish, they were leaked to laetrile advocates, resulting in significant public attention.[27]
To expand on Sugiura's results, MSKCC researchers conducted a controlled experiment in which they injected some mice with laetrile (as Sugiura had done) and others with placebo. Sugiura, who was unaware of which mice had received laetrile, performed the pathologic analysis. In this controlled, blinded follow-up of Sugiura's initial uncontrolled experiment, laetrile showed no more activity than placebo.[27]
Subsequently, laetrile was tested on 14 tumor systems without evidence of effectiveness. Given this collection of results, MSKCC concluded that "laetrile showed no beneficial effects."[27] Mistakes in the MSKCC press release were highlighted by a group of laetrile proponents led by Ralph Moss, former public affairs official of MSKCC who was fired following his appearance at a press conference accusing the hospital of covering up the benefits of laetrile.[28] These mistakes were considered scientifically inconsequential, but Nicholas Wade in Science stated that "even the appearance of a departure from strict objectivity is unfortunate."[27] The results from these studies were published all together.[29]
Subsequent clinical studies
A 2006 systematic review by the Cochrane Collaboration concluded: "The claim that [l]aetrile has beneficial effects for cancer patients is not supported by data from controlled clinical trials. This systematic review has clearly identified the need for randomised or controlled clinical trials assessing the effectiveness of [l]aetrile or amygdalin for cancer treatment."[30] Given the lack of evidence, laetrile has not been approved by the U.S. Food and Drug Administration.[14]
The U.S. National Institutes of Health evaluated the evidence separately and concluded that clinical trials of amgydalin showed little or no effect against cancer.[22] For example, a 1982 trial of 175 patients found that tumor size had increased in all but one patient.[31] The authors reported that "the hazards of amygdalin therapy were evidenced in several patients by symptoms of cyanide toxicity or by blood cyanide levels approaching the lethal range."[6]
The study concluded "Patients exposed to this agent should be instructed about the danger of cyanide poisoning, and their blood cyanide levels should be carefully monitored. Amygdalin (Laetrile) is a toxic drug that is not effective as a cancer treatment".
Advocacy and legality
Although laetrile is widely considered quackery in the medical community, advocates for laetrile dispute this label, asserting that there is a conspiracy between the U.S. Food and Drug Administration, the pharmaceutical industry and the medical community, including the American Medical Association and the American Cancer Society, to exploit the American people, and especially cancer patients.[32] Some North American cancer patients have traveled to Mexico for treatment with the substance, allegedly under the auspices of Dr. Ernesto Contreras.[33] Actor Steve McQueen died in Mexico following cancer treatment with laetrile and surgery to remove a stomach tumor while undergoing treatment for peritoneal mesothelioma (a cancer that attacks the lining of the abdomen) under the care of William D. Kelley, a dentist and orthodontist who devised a supposed cancer treatment based on laetrile.[34]
Laetrile advocates in the United States include Dean Burk (now deceased), a former chief chemist of the National Cancer Institute cytochemistry laboratory,[35] and national arm wrestling champion Jason Vale, who claimed that his kidney and pancreatic cancers were cured by eating apricot seeds. Vale was convicted in 2003 for, among other things, marketing laetrile.[36] The court also found that Vale, who had made at least $500,000 from his illegal sales of laetrile, had fraudulently marketed the substance.[37]
The US Food and Drug Administration continues to seek jail sentences for vendors marketing laetrile for cancer treatment, calling it a "highly toxic product that has not shown any effect on treating cancer."[38]
See also
- United States v. Rutherford, 442 U.S. 544 (1979). Finding: The Federal Food, Drug, and Cosmetic Act makes no express exception for drugs used by the terminally ill and no implied exemption is necessary to attain congressional objectives or to avert an unreasonable reading of the terms "safe" and "effective" in 201 (p) (1).
References
 This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). "Amygdalin". Encyclopædia Britannica (11th ed.). Cambridge University Press. http://en.wikisource.org/wiki/1911_Encyclop%C3%A6dia_Britannica/Amygdalin.
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). "Amygdalin". Encyclopædia Britannica (11th ed.). Cambridge University Press. http://en.wikisource.org/wiki/1911_Encyclop%C3%A6dia_Britannica/Amygdalin.
- ^ "A chronology of significant historical developments in the biological sciences". Botany Online Internet Hypertextbook. University of Hamburg, Department of Biology. 2002-08-18. http://www.biologie.uni-hamburg.de/b-online/e01/geschichte.htm. Retrieved 2007-08-06.
- ^ Swain E, Poulton JE (October 1994). "Utilization of Amygdalin during Seedling Development of Prunus serotina". Plant physiology 106 (2): 437–445. doi:10.1104/pp.106.2.437. PMC 159548. PMID 12232341. http://www.plantphysiol.org/cgi/pmidlookup?view=long&pmid=12232341.
- ^ a b c d Lerner IJ (1981). "Laetrile: a lesson in cancer quackery". CA Cancer J Clin 31 (2): 91–5. doi:10.3322/canjclin.31.2.91. PMID 6781723. http://caonline.amcancersoc.org/cgi/reprint/31/2/91.
- ^ Ellison NM, Byar DP, Newell GR (September 1978). "Special report on Laetrile: the NCI Laetrile Review. Results of the National Cancer Institute's retrospective Laetrile analysis". N. Engl. J. Med. 299 (10): 549–52. doi:10.1056/NEJM197809072991013. PMID 683212.
- ^ Moertel CG, Ames MM, Kovach JS, Moyer TP, Rubin JR, Tinker JH (February 1981). "A pharmacologic and toxicological study of amygdalin". JAMA 245 (6): 591–4. doi:10.1001/jama.245.6.591. PMID 7005480.
- ^ a b Moertel CG, Fleming TR, Rubin J (January 1982). "A clinical trial of amygdalin (Laetrile) in the treatment of human cancer". N. Engl. J. Med. 306 (4): 201–6. doi:10.1007/s00520-006-0168-9. PMID 7033783. http://content.nejm.org/cgi/content/abstract/306/4/201.
- ^ O'Brien B, Quigg C, Leong T (October 2005). "Severe cyanide toxicity from 'vitamin supplements'". Eur J Emerg Med 12 (5): 257–8. doi:10.1097/00063110-200510000-00014. PMID 16175068.
- ^ a b c Herbert V (May 1979). "Laetrile: the cult of cyanide. Promoting poison for profit". Am. J. Clin. Nutr. 32 (5): 1121–58. PMID 219680. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=219680.
- ^ Lerner IJ (February 1984). "The whys of cancer quackery". Cancer 53 (3 Suppl): 815–9. doi:10.1002/1097-0142(19840201)53:3+<815::AID-CNCR2820531334>3.0.CO;2-U. PMID 6362828.
- ^ Nightingale SL (1984). "Laetrile: the regulatory challenge of an unproven remedy". Public Health Rep 99 (4): 333–8. PMC 1424606. PMID 6431478. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1424606.
- ^ F. Wöhler, J. Liebig (1837). "Ueber die Bildung des Bittermandelöls". Annalen der Pharmacie 22 (1): 1–24. doi:10.1002/jlac.18370220102.
- ^ J. W. Walker, V. K. Krieble (1909). "The hydrolysis of amygdalin by acids. Part I". Journal of the Chemical Society 95 (11): 1369–77. doi:10.1039/CT9099501369.
- ^ a b Fenselau C, Pallante S, Batzinger RP (November 1977). "Mandelonitrile beta-glucuronide: synthesis and characterization". Science 198 (4317): 625–7. doi:10.1126/science.335509. PMID 335509. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=335509.
- ^ a b c What is laetrile?, National Cancer Institute, Retrieved on 14 January 2007
- ^ Collins, Peter M. (2005). Dictionary of Carbohydrates. Boca Raton: CRC Press. p. 678. ISBN 0-8493-3829-8. http://books.google.com/?id=LPAH57E2V2sC&pg=PA678&dq=laetrile+Mp#v=onepage&q=laetrile%20Mp&f=false.
- ^ a b Laetrile data in PubChem.
- ^ Hoffman, E. J. (1999). Cancer and the search for selective biochemical inhibitors. Boca Raton: CRC Press. p. 76. ISBN 0-8493-9118-0. http://books.google.com/books?id=WiIVBWKJhYwC&pg=PA66&dq=methods+in+Plant+Biochemistry,+Alkaloids+and+Sulfur+Compounds.&cd=3#v=snippet&q=%22laetrile%20and%20amygdalin%2C%20which%22&f=false.
- ^ Newmark J, Brady RO, Grimley PM, Gal AE, Waller SG, Thistlethwaite JR (October 1981). "Amygdalin (Laetrile) and prunasin beta-glucosidases: distribution in germ-free rat and in human tumor tissue". Proc. Natl. Acad. Sci. U.S.A. 78 (10): 6513–6. doi:10.1073/pnas.78.10.6513. PMC 349070. PMID 6796962. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=349070.
- ^ Newton GW, Schmidt ES, Lewis JP, Conn E, Lawrence R (February 1981). "Amygdalin Toxicity Studies in Rats Predict Chronic Cyanide Poisoning in Humans". West. J. Med. 134 (2): 97–103. PMC 1272529. PMID 7222669. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1272529.
- ^ Shragg TA, Albertson TE, Fisher CJ (January 1982). "Cyanide poisoning after bitter almond ingestion". West. J. Med. 136 (1): 65–9. PMC 1273391. PMID 7072244. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1273391.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 91-93.
- ^ a b c Laetrile/Amygdalin - National Cancer Institute
- ^ E Krebs (May 23, 1961). Hexuronic acid derivatives. http://www.freepatentsonline.com/2985664.html.
- ^ Carpenter, Daniel (2010). Reputation and Power: Organizational Image and Pharmaceutical Regulation at the FDA. Princeton: Princeton University Press.. Princeton: Princeton University Press. ISBN 0691141800.
- ^ Kennedy, Donald (1977). "Laetrile: The Commissioner's Decision" (PDF). Federal Register Docket No. 77-22310. http://www.cancertreatmentwatch.org/q/laetrile/commissioner.pdf.
- ^ American Cancer Society (1991). "Unproven methods of cancer management. Laetrile". CA Cancer J Clin 41 (3): 187–92. doi:10.3322/canjclin.41.3.187. PMID 1902140. http://caonline.amcancersoc.org/cgi/pmidlookup?view=long&pmid=1902140.
- ^ a b c d e Wade N (December 1977). "Laetrile at Sloan-Kettering: A Question of Ambiguity". Science 198 (4323): 1231–4. doi:10.1126/science.198.4323.1231. PMID 17741690.
- ^ Budiansky, Stephen (1995-07-09). "Cures or Quackery: How Senator Harkin shaped federal research on alternative medicine". US News and World Report. http://www.usnews.com/usnews/culture/articles/950717/archive_032434.htm. Retrieved 2009-11-07.
- ^ Stock CC, Tarnowski GS, Schmid FA, Hutchison DJ, Teller MN (1978). "Antitumor tests of amygdalin in transplantable animal tumor systems". J Surg Oncol 10 (2): 81–8. doi:10.1002/jso.2930100202. PMID 642516. http://www3.interscience.wiley.com/journal/112720432/issue.
Stock CC, Martin DS, Sugiura K (1978). "Antitumor tests of amygdalin in spontaneous animal tumor systems". J Surg Oncol 10 (2): 89–123. doi:10.1002/jso.2930100203. PMID 347176. - ^ Milazzo S, Ernst E, Lejeune S, Schmidt K (2006). Milazzo, Stefania. ed. "Laetrile treatment for cancer". Cochrane Database Syst Rev (2): CD005476. doi:10.1002/14651858.CD005476.pub2. PMID 16625640.
- ^ http://www.cancerhelp.org.uk/about-cancer/treatment/complementary-alternative/therapies/laetrile
- ^ Editors of Consumer Reports Books (1980). "Laetrile: the Political Success of a Scientific Failure". Health Quackery. Vernon, New York: Consumers Union. pp. 16–40. ISBN 0-89043-014-4
- ^ Moss RW (March 2005). "Patient perspectives: Tijuana cancer clinics in the post-NAFTA era". Integr Cancer Ther 4 (1): 65–86. doi:10.1177/1534735404273918. PMID 15695477.
- ^ Lerner, Barron H. (2005-11-15). "McQueen's Legacy of Laetrile". New York Times. http://www.nytimes.com/2005/11/15/health/15essa.html?ex=1289710800&en=8059981c17deec5d&ei=5088. Retrieved 2010-04-23.
- ^ "Dean Burk, 84, Noted Chemist At National Cancer Institute, Dies". Washington Post. 9 October 1988. http://www.highbeam.com/doc/1P2-1283487.html.
- ^ Brian S. McWilliams (2005). Spam kings: the real story behind the high-rolling hucksters pushing porn, pills and @*#?% enlargements. Sebastopol, CA: O'Reilly. ISBN 0-596-00732-9. http://books.google.com/?id=h1K1bIcfhjcC&pg=PA237&dq=Jason+Vale.
- ^ "New York Man Sentenced to 63 Months for Selling Fake Cancer Cure". Medical News Today. 22 June 2004. http://www.medicalnewstoday.com/articles/9825.php. Retrieved 8 July 2010.
- ^ US FDA (June 22, 2004). Lengthy Jail Sentence for Vendor of Laetrile—A Quack Medication to Treat Cancer Patients. FDA News
External links
- Laetrile/Amygdalin information from the U.S. National Cancer Institute
- Laetrile treatment for cancer, from the Cochrane Library
- Food and Drug Administration Commissioner's Decision on Laetrile
- The Rise and Fall of Laetrile
Bond Geometry Glycone Aglycone biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iCategories:- Glycosides
- Almonds
- Alternative cancer treatments
- Plant toxins
- Nitriles
- O[C@@H]3[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]3OC[C@H]2O[C@@H](OC(C#N)c1ccccc1)[C@H](O)[C@@H](O)[C@@H]2O
Wikimedia Foundation. 2010.