- Asymmetric induction
-
Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment.[1] Asymmetric induction is a key element in asymmetric synthesis.
Asymmetric induction was introduced by Emil Fischer based on his work on carbohydrates.[2] Several types of induction exist.
Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.
Contents
Carbonyl 1,2 asymmetric induction
Several models exist to describe chiral induction at carbonyl carbons during nucleophilic additions. These models are based on a combination of steric and electronic considerations and are often in conflict with each other. Models have been devised by Cram (1952), Cornforth (1959), Felkin (1969) and others.
Cram's rule
The Cram's rule of asymmetric induction developed by Donald J. Cram in 1952[3] is an early concept relating to the prediction of stereochemistry in certain acyclic systems. In full the rule is:
In certain non-catalytic reactions that diastereomer will predominate, which could be formed by the approach of the entering group from the least hindered side when the rotational conformation of the C-C bond is such that the double bond is flanked by the two least bulky groups attached to the adjacent asymmetric center.
The rule indicates that the presence of an asymmetric center in a molecule induces the formation of an asymmetric center adjacent to it based on steric hindrance.
In his 1952 publication Cram presented a large number of reactions described in the literature for which the conformation of the reaction products could be explained based on this rule and he also described an elaborate experiment (scheme 1) making his case.
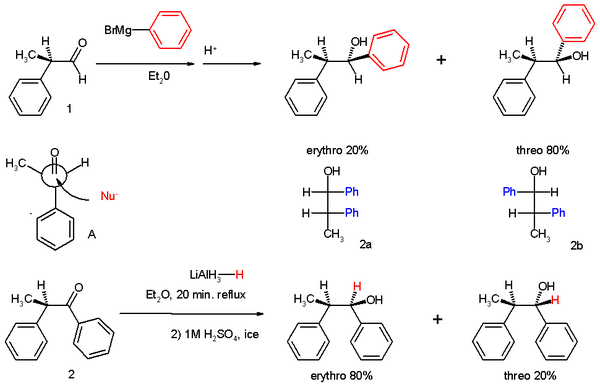
The experiments involved two reactions. In experiment one 2-phenylpropionaldehyde (1, racemic but (R)-enantiomer shown) was reacted with the Grignard reagent of bromobenzene to 1,2-diphenyl-1-propanol (2) as a mixture of diastereomers, predominantly the threo isomer (see for explanation the Fischer projection).
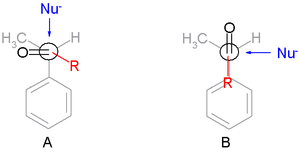
The preference for the formation of the threo isomer can be explained by the rule stated above by having the active nucleophile in this reaction attacking the carbonyl group from the least hindered side (see Newman projection A) when the carbonyl is positioned in a staggered formation with the methyl group and the hydrogen atom, which are the two smallest substituents creating a minimum of steric hindrance, in a gauche orientation and phenyl as the most bulky group in the anti conformation.
The second reaction is the organic reduction of 1,2-diphenyl-1-propanone 2 with lithium aluminium hydride, which results in the same reaction product as above but now with preference for the erythro isomer (2a). Now a hydride anion (H-) is the nucleophile attacking from the least hindered side (imagine hydrogen entering from the paper plane).
In the original 1952 publication, additional evidence was obtained for the structural assignment of the reaction products by applying them to a Chugaev elimination, wherein the threo isomer reacts to the cis isomer of -α-methyl-stilbene and the erythro isomer to the trans version.
Felkin model
The Felkin model (1968) named after Hugh Felkin also predicts the stereochemistry of nucleophilic addition reactions to carbonyl groups[4]. Felkin argued that the Cram model suffered a major drawback: an eclipsed conformation in the transition state between the carbonyl substituent (the hydrogen atom in aldehydes) and the largest α-carbonyl substituent. He demonstrated that by increasing the steric bulk of the carbonyl substituent from methyl to ethyl to isopropyl to isobutyl, the stereoselectivity also increased, which is not predicted by Cram's rule:
The Felkin rules are:
- The transition states are reactant-like.
- Torsional strain (Pitzer strain) involving partial bonds (in transition states) represents a substantial fraction of the strain between fully formed bonds, even when the degree of bonding is quite low. The conformation in the TS is staggered and not eclipsed with the substituent R skew with respect to two adjacent groups one of them the smallest in TS A.
- The main steric interactions involve those around R and the nucleophile but not the carbonyl oxygen atom.
- A polar effect or electronic effect stabilizes a transition state with maximum separation between the nucleophile and an electron-withdrawing group. For instance haloketones do not obey Cram's rule, and, in the example above, replacing the electron-withdrawing phenyl group by a cyclohexyl group reduces stereoselectivity considerably.
Felkin-Anh model
The Felkin-Anh model[5] is an extension of the Felkin model that incorporates improvements suggested by Nguyen T. Anh and O. Eisenstein to correct for two key weaknesses in Felkin's model. The first weakness addressed was the statement by Felkin of a strong polar effect in nucleophilic addition transition states, which leads to the complete inversion of stereochemistry by SN2 reactions, without offering justifications as to why this phenomenon was observed. Anh's solution was to offer the antiperiplanar effect as a consequence of asymmetric induction being controlled by both substituent and orbital effects[6][7]. In this effect, the best nucleophile acceptor σ* orbital is aligned parallel to both the π and π* orbitals of the carbonyl, which provide stabilization of the incoming anion.

The second weakness in the Felkin Model was the assumption of substituent minimization around the carbonyl R, which cannot be applied to aldehydes.

Incorporation of Bürgi–Dunitz angle[8][9] ideas allowed Anh to postulate a non-perpendicular attack by the nucleophile on the carbonyl center, anywhere from 95o to 105o relative to the oxygen-carbon double bond, favoring approach closer to the smaller substituent and thereby solve the problem of predictability for aldehydes[6][10][11].

Anti–Felkin selectivity
Though the Cram and Felkin–Anh models differ in the conformers considered and other assumptions, they both attempt to explain the same basic phenomenon: the preferential addition of a nucleophile to the most sterically favored face of a carbonyl moiety. However, many examples exist of reactions that display stereoselectivity opposite of what is predicted by the basic tenets of the Cram and Felkin–Anh models. Although both of the models include attempts to explain these reversals, the products obtained are still referred to as "anti-Felkin" products. One of the most common examples of altered asymmetric induction selectivity requires an α-carbon substituted with a component with Lewis base character (i.e. O, N, S, P substituents). In this situation, if a Lewis acid such as Al-iPr2 or Zn2+ is introduced, a bidentate chelation effect can be observed. This locks the carbonyl and the Lewis base substituent in an eclipsed conformation, and the nucleophile will then attack from the side with the smallest free α-carbon substituent[12]. If the chelating R group is identified as the largest, this will result in an "anti-Felkin" product.

This stereoselective control was recognized and discussed in the first paper establishing the Cram model, causing Cram to assert that his model requires non-chelating conditions[13]. An example of chelation control of a reaction can be seen here, from a 1987 paper that was the first to directly observe such a "Cram-chelate" intermediate[14], vindicating the model:

Here, the methyl titanium chloride forms a Cram-chelate. The methyl group then dissociates from titanium and attacks the carbonyl, leading to the anti-Felkin diastereomer.
A non-chelating electron-withdrawing substituent effect can also result in anti-Felkin selectivity. If a substituent on the α-carbon is sufficiently electron withdrawing, the nucleophile will add anti- relative to the electron withdrawing group, even if the substituent is not the largest of the 3 bonded to the α-carbon. Each model offers a slightly different explanation for this phenomenon. A polar effect was postulated by the Cornforth model[15] and the original Felkin model[16], which placed the EWG substituent and incoming nucleophile anti- to each other in order to most effectively cancel the dipole moment of the transition structure.

This Newman projection illustrates the Cornforth and Felkin transition state that places the EWG anti- to the incoming nucleophile, regardless of its steric bulk relative to RS and RL.
The improved Felkin–Anh model, as discussed above, makes a more sophisticated assessment of the polar effect by considering molecular orbital interactions in the stabilization of the preferred transition state. A typical reaction illustrating the potential anti-Felkin selectivity of this effect, along with its proposed transition structure, is pictured below:

Carbonyl 1,3 asymmetric induction
It has been observed that the stereoelectronic environment at the β-carbon of can also direct asymmetric induction. A number of predictive models have evolved over the years to define the stereoselectivity of such reactions.
Chelation model
According to Reetz, the Cram-chelate model for 1,2-inductions can be extended to predict the chelated complex of a β-alkoxy aldehyde and metal. The nucleophile is seen to attack from the less sterically hindered side and anti- to the substituent Rβ, leading to the anti-adduct as the major product[17].

To make such chelates, the metal center must have at least two free coordination sites and the protecting ligands should form a bidentate complex with the Lewis acid.
Non-chelation model
Cram–Reetz model
Cram and Reetz demonstrated that 1,3-stereocontrol is possible if the reaction proceeds through an acyclic transition state. The reaction of β-alkoxy aldehyde with allyltrimethylsilane showed good selectivity for the anti-1,3-diol, which was explained by the Cram polar model. The polar benzyloxy group is oriented anti to the carbonyl to minimize dipole interactions and the nucleophile attacks anti- to the bulkier (RM) of the remaining two substituents[18][19].

Evans model
More recently, Evans presented a different model for nonchelate 1,3-inductions. In the proposed transition state, the β-stereocenter is oriented anti- to the incoming nucleophile, as seen in the Felkin–Anh model. The polar X group at the β-stereocenter is placed anti- to the carbonyl to reduce dipole interactions, and Rβ is placed anti- to the aldehyde group to minimize the steric hindrance. Consequently, the 1,3-anti-diol would be predicted as the major product[20].

Carbonyl 1,2 and 1,3 asymmetric induction
If the substrate has both an α- and β-stereocenter, the Felkin–Anh rule (1,2-induction) and the Evans model (1,3-induction) should considered at the same time. If these two stereocenters have an anti- relationship, both models predict the same diastereomer (the stereoreinforcing case).

However, in the case of the syn-substrate, the Felkin–Anh and the Evans model predict different products (non-stereoreinforcing case). It has been found that the size of the incoming nucleophile determines the type of control exerted over the stereochemistry. In the case of a large nucleophile, the interaction of the α-stereocenter with the incoming nucleophile becomes dominant; therefore, the Felkin product is major one. Smaller nucleophiles, on the other hand, result in 1,3 control determining the asymmetry[21].

See Also
- Macrocyclic Stereocontrol
References
- ^ IUPAC Gold Book definition Link
- ^ Asymmetric Synthesis of Natural Products, Ari Koskinen ISBN 0-471-93848-3
- ^ Studies in Stereochemistry. X. The Rule of "Steric Control of Asymmetric Induction" in the Syntheses of Acyclic Systems Donald J. Cram, Fathy Ahmed Abd Elhafez J. Am. Chem. Soc.; 1952; 74(23); 5828–5835. Abstract
- ^ Torsional strain involving partial bonds. The stereochemistry of the lithium aluminium hydride reduction of some simple open-chain ketones Marc Chérest, Hugh Felkin and Nicole Prudent Tetrahedron Letters Volume 9, Issue 18, 1968, Pages 2199-2204 doi:10.1016/S0040-4039(00)89719-1
- ^ It bears mentioning that in Vietnamese, the surname is given first, and so this would be better called the Felkin-Nguyen Model.
- ^ a b Anh, N. T.; Eisenstein, O. Nouv. J. Chim. 1977, 1, 61.
- ^ Anh, N. T.; Eisenstein, O.; Lefour, J-M.; Dau, M-E. J. Am. Chem. Soc. 1973, 95, 6146.
- ^ Bürgi, H. B.; Dunitz, J. D.; Shefter, E. J. Am. Chem. Soc. 1973, 95, 5065.
- ^ Bürgi, H. B.; Dunitz, J. D.; Lehn, J. M.; Wipff, G. Tetrahedron 1974, 30, 1563.
- ^ Anh, N. T.; Eisenstein, O. Tetrahedron Lett. 1976, 155.
- ^ Anh, N. T. Top. Curr. Chem. 1980, 88, 146.
- ^ Mengel A., Reiser O.Chem. Rev., 1999, 99 (5), 1191–1224.
- ^ Cram DJ, Elhafez FA. J. Am. Chem. Soc.; 1952; 74(23); 5828–5835.
- ^ Reetz MT, Hullmann M, Seitz T. Angew. Chem. Int. Ed. Engl. 1987. 26, 477–480.
- ^ Cornforth JW, Cornforth MRH, Mathew KK. J. Chem.Soc. 1959, 112–127.
- ^ Cherest M, Felkin H, Prudent N. Tetrahedron Lett. 1968, 18, 2199–2204.
- ^ Reetz, M.T.; Jung, A. J. Am. Chem. Soc, 1983, 105, 4833.
- ^ Leitereg, T.J.; Cram, D.J. J. Am. Chem. Soc. 1968, 90, 4011.
- ^ Reetz. M.T.; Kesseler, K.; Jung, A. Tetrahedron Lett. 1984, 25, 729.
- ^ Evans, D.A.; Duffy, J.L.; Dart, M.J. Tetrahedron Lett. 1994, 35, 8537.
- ^ Evans, D.A.; Dart, M.J.; Duffy, J.L.; Yang, M.G. J .Am. Chem. Soc. 1996, 118, 4322.
External links
- The Evolution of Models for Carbonyl Addition Evans Group Afternoon Seminar Sarah Siska February 9, 2001
Concepts in asymmetric synthesis Chirality types Chirality · Stereocenter · Planar chirality · Chiral ligand · Axial chirality · Supramolecular chirality · Inherent chiralityChiral molecules Stereoisomer · Enantiomer · Diastereomer · Meso compound · Enantiomeric excess · Diastereomeric excess ·Analysis Optical rotation · Chiral derivatizing agents · NMR spectroscopy of stereoisomers · Ultraviolet-visible spectroscopy of stereoisomersChiral resolution Recrystallization · Kinetic resolution · Chiral column chromatography · Diastereomeric recrystallizationReactions Asymmetric induction · Chiral pool synthesis · Chiral auxiliaries · Asymmetric catalysis · Organocatalysis · BiocatalysisCategories:
Wikimedia Foundation. 2010.