- Nucleophilic addition
-
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile.[1]
Addition reactions are limited to chemical compounds that have multiple-bonded atoms:
- molecules with carbon – hetero multiple bonds like carbonyls, imines or nitriles
- molecules with carbon – carbon double bonds or triple bonds.
Contents
Addition to carbon – hetero double bonds
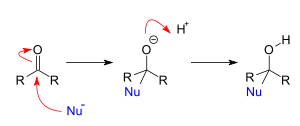
Addition reactions of a nucleophile to carbon – hetero double bonds such as C=O or CN triple bond show a wide variety. These bonds are polar (have a large difference in electronegativity between the two atoms) consequently carbon carries a partial positive charge. This makes this atom the primary target for the nucleophile.
This type of reaction is also called a 1,2 nucleophilic addition. The stereochemistry of this type of nucleophilic attack is not an issue, when both alkyl substituents are dissimilar and there are not any other controlling issues such as chelation with a Lewis acid, the reaction product is a racemate. Addition reactions of this type are numerous. When the addition reaction is accompanied by an elimination the reaction type is nucleophilic acyl substitution or an addition-elimination reaction.
Carbonyls
With a carbonyl compound as an electrophile, the nucleophile can be:
- water in hydration to a geminal diol (hydrate)
- an alcohol in acetalisation to an acetal
- a hydride in reduction to an alcohol
- an amine with formaldehyde and a carbonyl compound in the Mannich reaction
- an enolate ion in an aldol reaction or Baylis–Hillman reaction
- an organometallic nucleophile in the Grignard reaction or the related Barbier reaction or a Reformatskii reaction
- ylides such as a Wittig reagent or the Corey–Chaykovsky reagent or α-silyl carbanions in the Peterson olefination
- a phosphonate carbanion in the Horner–Wadsworth–Emmons reaction
- a pyridine zwitterion in the Hammick reaction
- an acetylide in the Favorskii reaction.
Nitriles
With nitrile electrophiles nucleophilic addition take place by:
- hydrolysis of a nitrile to an amide or a carboxylic acid
- organozinc nucleophiles in the Blaise reaction
- alcohols in the Pinner reaction.
- the (same) nitrile α-carbon in the Thorpe reaction. The intramolecular version is called the Thorpe–Ziegler reaction.
Imines and other
With imine electrophiles nucleophilic addition take place by:
- hydrides to amines in the Eschweiler–Clarke reaction
- water to carbonyls in the Nef reaction.
With miscellaneous electrophiles:
- addition of an alcohol to an isocyanate to form a carbamate.
Nucleophiles attack carbonyl centers from a specific angle called the Bürgi–Dunitz angle.
Addition to carbon – carbon double bonds
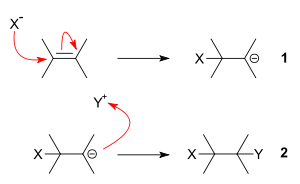
The driving force for the addition to alkenes is the formation of a nucleophile X− that forms a covalent bond with an electron-poor unsaturated system -C=C- (step 1). The negative charge on X is transferred to the carbon – carbon bond.
In step 2 the negatively charged carbanion combines with (Y) that is electron-poor to form the second covalent bond.
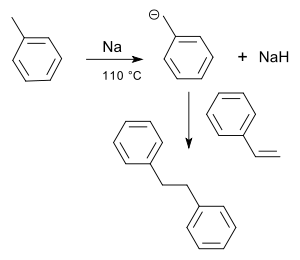
Ordinary alkenes are not susceptible to a nucleophilic attack (apolar bond). Styrene reacts in toluene with sodium to 1,3-diphenylpropane [2] through the intermediate carbanion:
Another exception to the rule is found in the Varrentrapp reaction. Fullerenes have unusual double bond reactivity and additions such has the Bingel reaction are more frequent.
When X is a carbonyl group like C=O or COOR or a cyanide group (CN), the reaction type is a conjugate addition reaction. The substituent X helps to stabilize the negative charge on the carbon atom by its inductive effect.
In addition when Y-Z is an active hydrogen compound the reaction is known as a Michael reaction.
Perfluorinated alkenes (alkenes that have all hydrogens replaced by fluorine) are highly prone to nucleophilic addition, for example by fluoride ion from caesium fluoride or silver(I) fluoride to give a perfluoroalkyl anion.
References
- ^ March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. ISBN 0-471-85472-7
- ^ Sodium-catalyzed Side Chain Aralkylation of Alkylbenzenes with Styrene Herman Pines, Dieter Wunderlich J. Am. Chem. Soc.; 1958; 80(22)6001–6004. doi:10.1021/ja01555a029
Categories:- Addition reactions
- Reaction mechanisms
Wikimedia Foundation. 2010.