- Caesium fluoride
-
Caesium fluoride 
 Caesium fluorideOther namesCesium fluoride
Caesium fluorideOther namesCesium fluorideIdentifiers CAS number 13400-13-0 
ChemSpider 24179 
RTECS number FK9650000 Jmol-3D images Image 1 - [F-].[Cs+]
Properties Molecular formula CsF Molar mass 151.90 g/mol Appearance white crystalline solid Density 4.115 g/cm3 Melting point 682 °C (955 K)
Boiling point 1251 °C (1524 K)
Solubility in water 367 g/100 ml (18 °C) Structure Crystal structure cubic, cF8 Space group Fm3m, No. 225 Coordination
geometryOctahedral Dipole moment 7.9 D Hazards MSDS External MSDS EU Index Not listed Flash point Non-flammable Related compounds Other anions Caesium chloride
Caesium bromide
Caesium iodideOther cations Lithium fluoride
Sodium fluoride
Potassium fluoride
Rubidium fluoride fluoride (verify) (what is:
fluoride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Caesium fluoride (cesium fluoride in North America), is an inorganic compound usually encountered as a hygroscopic white solid. It is more soluble and more readily dissociated than sodium fluoride or potassium fluoride. It is available in anhydrous form, and if water has been absorbed it is easy to dry by heating at 100 °C for two hours in vacuo.[1] Like all soluble fluorides, it is mildly basic. A notable fact about this compound is that it is the most ionic compound. Caesium has the lowest electronegativity and fluorine has the highest electronegativity.
Contents
Synthesis and properties
Caesium fluoride is prepared by the action of hydrofluoric acid on caesium hydroxide or caesium carbonate, followed by removal of water.
Caesium fluoride reacts usually as a source of fluoride ion, F-. It therefore undergoes all of the usual reactions associated with soluble fluorides, for example:[2]
- 2 CsF + CaCl2 → 2 CsCl + CaF2
Crystal structure
Caesium fluoride has the halite structure, which means that the Cs+ and F− pack in a cubic closest packed array as do Na+ and Cl− in sodium chloride. Caesium cations are larger than fluoride anions, whereas in the lithium, sodium, potassium, and rubidium halides, the cations are smaller than the anion. [2][3]
Applications
In organic synthesis
Being highly dissociated it is a more reactive source of fluoride than related salts. CsF is less hygroscopic alternative to tetra-n-butylammonium fluoride (TBAF) and TAS-fluoride (TASF) when anhydrous "naked" fluoride ion is needed.
As a base
As with other soluble fluorides, CsF is moderately basic, because HF is a weak acid. The low nucleophilicity of fluoride means it can be a useful base in organic chemistry.[2]Caesium fluoride is a useful base in organic chemistry, due the fact that fluoride ion is a relatively poor nucleophile. CsF gives higher yields in Knoevenagel condensation reactions than KF or NaF.[4]
Formation of C-F bonds
Caesium fluoride is also a popular source of fluoride in organofluorine chemistry. For example, CsF reacts with hexafluoroacetone to form a caesium perfluoroalkoxide salt, which is stable up to 60 °C, unlike the corresponding sodium or potassium salt. It will convert electron-deficient aryl chlorides to aryl fluorides (halex reaction).[5]
Deprotection agent
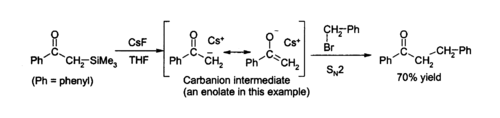
Due to the strength of the Si–F bond, fluoride ion is useful for desilylation reactions (removal of Si groups) in organic chemistry; caesium fluoride is an excellent source of anhydrous fluoride for such reactions. Removal of silicon groups (desilylation) is a major application for CsF in the laboratory, as its anhydrous nature allows clean formation of water-sensitive intermediates. Solutions of caesium fluoride in THF or DMF attack a wide variety of organosilicon compounds to produce an organosilicon fluoride and a carbanion, which can then react with electrophiles,[3] for example:[4]
Desilylation is also useful for the removal of silyl protecting groups.[6]
Other uses
Single crystals of the salt are transparent into the deep infrared. For this reason it is sometimes used as the windows of cells used for infrared spectroscopy.
Precautions
Like other soluble fluorides, CsF is moderately toxic.[7] Contact with acid should be avoided, as this forms highly toxic/corrosive hydrofluoric acid. Caesium ion (Cs+), or caesium chloride, is generally not considered toxic.[8]
References
- ^ Friestad, G. K.; Branchaud, B. P. (1999). Reich, H. J.; Rigby, J. H.. ed. Handbook of Reagents for Organic Synthesis: Acidic and Basic Reagents. New York: Wiley. pp. 99–103.
- ^ a b c Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, Pergamon Press, Oxford, UK, 1984.
- ^ a b Handbook of Chemistry and Physics, 71st edition, CRC Press, Ann Arbor, Michigan, 1990.
- ^ a b Fiorenza, M; Mordini, A; Papaleo, S; Pastorelli, S; Ricci, A (1985). "Fluoride ion induced reactions of organosilanes: the preparation of mono and dicarbonyl compounds from β-ketosilanes". Tetrahedron Letters 26 (6): 787. doi:10.1016/S0040-4039(00)89137-6.
- ^ F. W. Evans, M. H. Litt, A. M. Weidler-Kubanek, F. P. Avonda (1968). "Reactions Catalyzed by Potassium Fluoride. 111. The Knoevenagel Reaction". Journal of Organic Chemistry 33 (5): 1837–1839. doi:10.1021/jo01269a028.
- ^ Adam P. Smith, Jaydeep J. S. Lamba, and Cassandra L. Fraser “Efficient Synthesis of Halomethyl-2,2'-bipyridines: 4,4'-Bis(chloromethyl)-2,2'-bipyridine” Organic Syntheses, Vol. 78, p. 82 (2002); Collected Volume 10, p.107 (2004).
- ^ MSDS Listing for cesium fluoride. www.hazard.com. MSDS Date: April 27, 1993. Retrieved on September 7, 2007.
- ^ "MSDS Listing for cesium chloride." www.jtbaker.com. MSDS Date: January 16, 2006. Retrieved on September 7, 2007.
External links
Caesium compounds Categories:- Fluorides
- Caesium compounds
- Metal halides
Wikimedia Foundation. 2010.