- Organosilicon
-
Organosilicon compounds are organic compounds containing carbon silicon bonds. Organosilicon chemistry is the corresponding science exploring their properties and reactivity.[1]
Like carbon, the organically bound silicon is tetravalent and tetrahedral. Carbon-silicon bonds are generally absent in biochemical processes,[2] although there are reports of their fleeting existence in a freshwater alga.[3] The first organosilicon compound, tetraethylsilane was discovered by Charles Friedel and James Crafts in 1863 by reaction of tetrachlorosilane with diethylzinc. The carbosilicon silicon carbide is an inorganic compound.
Contents
Organosilanes
Carbon–silicon bonds compared to carbon–carbon bonds are longer (186 pm vs. 154 pm) and weaker with bond dissociation energy 451 kJ/mol vs. 607 kJ/mol [4]. The C–Si is somewhat polarized towards carbon due to its higher electronegativity (C 2.55 vs Si 1.90). One manifestation of bond polarization in organosilanes is found in the Sakurai reaction. In oxidative couplings silicon is represented by the Hiyama coupling. Certain alkyl silanes can be oxidized to an alcohol in the Fleming–Tamao oxidation.
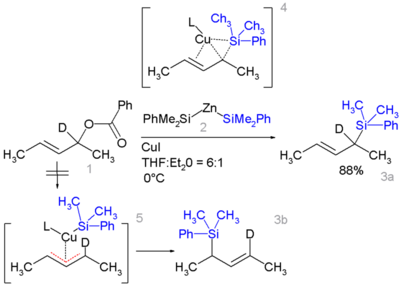
Certain allyl silanes can be prepared from allylic ester such as 1 and monosilylcopper compounds such as 2 in [5] [6].
In this reaction type silicon polarity is reversed in a chemical bond with zinc and a formal allylic substitution on the benzoyloxy group takes place.
The chemistry of silanes such as tetramethylsilane is comparable to that of alkanes in many aspects such as thermal stability. The β-silicon effect describes the stabilizing effect of a β-silicon atom on a carbocation with many implications for reactivity.
Siloxides
More notably bonds of silicon to oxygen are much shorter and stronger (809 compared to 538 kJ/mol) than that of those of carbon to oxygen. The polarization in this bond increases towards oxygen. Examples are silyl acetals RR'Si(OR)2, the silanols, the siloxanes and the polymeric polysiloxanes. Silyl ethers are extensively used as protective groups for alcohols. Only silicon bonds to fluorine are stronger and that is why the fluorine source TASF (or more commonly TBAF) is useful in deprotection. The favorable formation of Si–O bonds drive many organic reactions such as the Brook rearrangement and Peterson olefination.
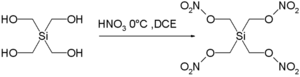
Another manifestation is the highly explosive nature of the silicon pendant Si(CH2ONO2)4 and Si(CH2N3)4 of pentaerythritol tetranitrate [7] [8]:
A single crystal of this compound, first synthesized in 2007 even detonates when in contact with a teflon spatula and in fact made full characterization impossible. Another contributor to its exothermic decomposition (inferred from much safer in silico experimentation) is the ability of silicon in its crystal phase to coordinate to two oxygen nitrito groups in addition to regular coordination to the four carbon atoms. This additional coordination would make formation of silicon dioxide (one of the decomposition products) more facile.
Silyl halides
Organosilyl halides are important reagents in organic chemistry notably trimethylsilyl chloride Me3SiCl. A classic method called the Flood reaction for the synthesis of this compound class is by heating hexaalkyldisiloxanes R3SiOSiR3 with concentrated sulfuric acid and a sodium halide [9]. Other relevant silyl halides are dichloromethylphenylsilane, dimethyldichlorosilane, methyltrichlorosilane, (4-aminobutyl)diethoxymethylsilane, trichloro(chloromethyl)silane, trichloro(dichlorophenyl)silane, trichloroethylsilane, trichlorophenylsilane and trimethylchlorosilane
Silyl hydrides
The silicon to hydrogen bond is longer than the C–H bond (148 compared to 105 pm) and weaker (299 compared to 338 kJ/mol). Hydrogen is more electronegative than silicon hence the naming convention of silyl hydrides. The parent compound SiH4 is called silane, and an example is phenylsilane. Silyl hydrides are very reactive and used as reducing agents for example PMHS.
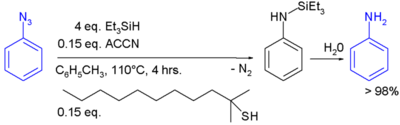
In one study triethylsilylhydride is used in the conversion of an phenyl azide to an aniline [10]:
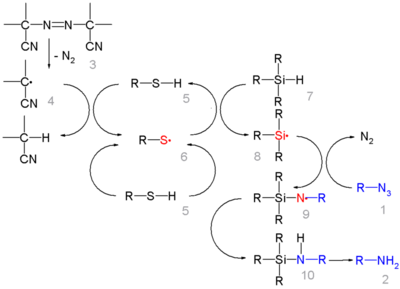
In this reaction ACCN is a radical initiator and an aliphatic thiol transfers radical character to the silylhydride. The triethylsilyl free radical then reacts with the azide with expulsion of nitrogen to a N-silylarylaminyl radical which abstracts a proton from a thiol completing the catalytic cycle:
Aqueous workup then gives aniline.
Silyl hydrides can even take up the reduction of robust molecules such as carbon dioxide (to methane) [11]:
Although it takes a very complex catalyst system.
Hydrosilylation
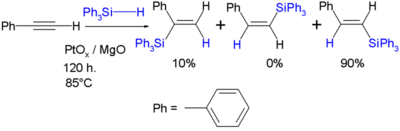
Silyl hydrides react with various unsaturated substrates such as alkenes, alkynes, imines, carbonyls and oximes to new organosilicon compounds in hydrosilylation. In the reaction of triphenylsilyl hydride with phenylacetylene the reaction product is a trans or cis or the geminal vinyl silane, for example [12]:
In the related silylmetalation, a metal replaces the hydrogen atom.
Silenes
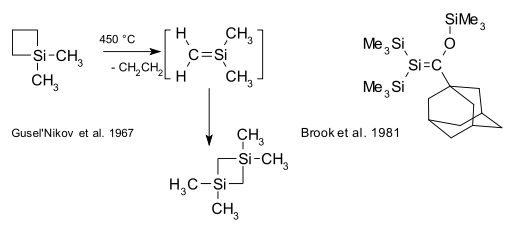
Organosilicon compounds, unlike their carbon counterparts, do not have a rich double bond chemistry due to the large difference in electronegativity [13]. Existing compounds with silene Si=C bonds (also known as alkylidenesilanes) are laboratory curiosities such as the silicon benzene analogue silabenzene. In 1967, Gusel'nikov and Flowers provided the first evidence for silenes from pyrolysis of dimethylsilacyclobutane [14]. The first stable (kinetically shielded) silene was reported in 1981 by Brook [15]
Disilenes have Si=Si double bonds and disilynes are silicon analogues of an alkyne. The first Silyne (with a silicon to carbon triple bond) was reported in 2010 [16]
Siloles
Siloles, also called silacyclopentadienes, are members of a larger class of compounds called metalloles, are the silicon analogs of pyrroles and are of current academic interest due to their electroluminescence and other electronic properties.[17] [18] Siloles are efficient in electron transport. They owe their low lying LUMO to a favorable interaction between the antibonding sigma silicon orbital with a antibonding pi orbital of the butadiene fragment.
Hypercoordinated silicon
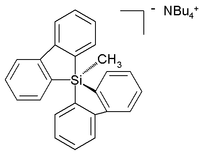
Unlike carbon, silicon compounds can be coordinated to five atoms as well in a group of compounds ranging from so-called silatranes, such as Phenylsilatrane, to a uniquely stable pentaorganosilicate [19]:
See also
- Compounds of carbon with period 3 elements: organoaluminum compounds, organosilicon compounds, organophosphorus compounds, organosulfur compounds,
- Compounds of carbon with other group 14 elements: organosilicon compounds, organogermanium compounds, organotin compounds, organolead compounds.
CH He CLi CBe CB CC CN CO CF Ne CNa CMg CAl CSi CP CS CCl CAr CK CCa CSc CTi CV CCr CMn CFe CCo CNi CCu CZn CGa CGe CAs CSe CBr CKr CRb CSr CY CZr CNb CMo CTc CRu CRh CPd CAg CCd CIn CSn CSb CTe CI CXe CCs CBa CHf CTa CW CRe COs CIr CPt CAu CHg CTl CPb CBi CPo CAt Rn Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo ↓ CLa CCe CPr CNd CPm CSm CEu CGd CTb CDy CHo CEr CTm CYb CLu Ac Th Pa CU Np Pu Am Cm Bk Cf Es Fm Md No Lr Chemical bonds to carbon Core organic chemistry Many uses in chemistry Academic research, but no widespread use Bond unknown / not assessed - silylenes, the carbene counterparts and silylenoids the carbenoid counterparts.
References
- ^ Silicon in Organic Synthesis Colvin, E. Butterworth: London 1981
- ^ Organosilicon Chemistry S. Pawlenko Walter de Gruyter New York 1986
- ^ Stephen D. Kinrade, Ashley-M. E. Gillson and Christopher T. G. Knight (2002), Silicon-29 NMR evidence of a transient hexavalent silicon complex in the diatom Navicula pelliculosa. J. Chem. Soc., Dalton Trans., 307 - 309, DOI: 10.1039/b105379p
- ^ Handbook of Chemistry and Physics, 81st Edition CRC Press ISBN 0-8493-0481-4
- ^ Mechanistic insight into copper-catalysed allylic substitutions with bis(triorganosilyl) zincs. Enantiospecific preparation of -chiral silanes Eric S. Schmidtmann and Martin Oestreich Chem. Commun., 2006, 3643 - 3645, doi:10.1039/b606589a
- ^ By isotopic desymmetrisation on the substrate (replacing hydrogen by deuterium) it can be demonstrated that the reaction proceeds not through the symmetrical π-allyl intermediate 5 which would give an equal mixture of 3a and 3b but through the Π-δ intermediate 4 resulting in 3a only, through an oxidative addition / reductive elimination step
- ^ The Sila-Explosives Si(CH2N3)4 and Si(CH2ONO2)4: Silicon Analogues of the Common Explosives Pentaerythrityl Tetraazide, C(CH2N3)4, and Pentaerythritol Tetranitrate, C(CH2ONO2)4 Thomas M. Klapötke, Burkhard Krumm, Rainer Ilg, Dennis Troegel, and Reinhold Tacke J. Am. Chem. Soc.; 2007; ASAP Web Release Date: 04-May-2007; (Article) doi:10.1021/ja071299p
- ^ Sila-Explosives Offer A Better Bang Stephen K. Ritter Chemical & Engineering News May 7 2007Link
- ^ Preparation of Triethylsilicon Halides E. A. Flood J. Am. Chem. Soc.; 1933; 55(4) pp 1735 - 1736; doi:10.1021/ja01331a504
- ^ Radical Reduction of Aromatic Azides to Amines with Triethylsilane Luisa Benati, Giorgio Bencivenni, Rino Leardini, Matteo Minozzi, Daniele Nanni, Rosanna Scialpi, Piero Spagnolo, and Giuseppe Zanardi Elumalai Palani., ''J. Org. Chem''.; 2006; 71(15) pp 5822 - 5825; (Note) doi:10.1021/jo060824k
- ^ From Carbon Dioxide to Methane: Homogeneous Reduction of Carbon Dioxide with Hydrosilanes Catalyzed by Zirconium-Borane Complexes Tsukasa Matsuo and Hiroyuki Kawaguchi J. Am. Chem. Soc.; 2006; 128(38) pp 12362 - 12363; doi:10.1021/ja0647250
- ^ Effect of the synthetic method of Pt/MgO in the hydrosilylation of phenylacetylene Eulalia Ramírez-Oliva, Alejandro Hernández, J. Merced Martínez-Rosales, Alfredo Aguilar-Elguezabal, Gabriel Herrera-Pérez, and Jorge Cervantesa Arkivoc 2006 (v) 126-136 Link
- ^ Silylenes, Silenes, and Disilenes: Novel Silicon-Based Reagents for Organic Synthesis? Henrik Ottosson and Patrick G. Steel Chem. Eur. J. 2006, 12, 1576 – 1585 doi:10.1002/chem.200500429
- ^ The thermal decomposition of 1,1-dimethyl-1-silacyclobutane and some reactions of an unstable intermediate containing a silicon–carbon double bond L. E. Gusel'Nikov and M. C. Flowers Chem. Commun. (London), 1967, 864 - 865, doi:10.1039/C19670000864
- ^ A solid silaethene: isolation and characterization Adrian G. Brook, Fereydon Abdesaken, Brigitte Gutekunst, Gerhard Gutekunst and R. Krishna Kallury J. Chem. Soc., Chem. Commun., 1981, 191 - 192, doi:10.1039/C39810000191
- ^ Gau, D., Kato, T., Saffon-Merceron, N., De Cózar, A., Cossío, F. and Baceiredo, A. (2010), Synthesis and Structure of a Base-Stabilized C-Phosphino-Si-Amino Silyne. Angewandte Chemie International Edition, 49: 6585–6588. doi:10.1002/anie.201003616
- ^ Direct synthesis of 2,5-dihalosiloles Organic Syntheses 2008, 85, 53-63 http://www.orgsynth.org/orgsyn/pdfs/V85P0053.pdf
- ^ Synthesis of new dipyridylphenylaminosiloles for highly emissive organic electroluminescent devices Laurent Aubouy, Philippe Gerbier, Nolwenn Huby, Guillaume Wantz, Laurence Vignau, Lionel Hirsch and Jean-Marc Jano New J. Chem., 2004, 28, 1086 - 1090, doi:10.1039/b405238b
- ^ Tetraalkylammonium pentaorganosilicates: the first highly stable silicates with five hydrocarbon ligands Sirik Deerenberg, Marius Schakel, Adrianus H. J. F. de Keijzer, Mirko Kranenburg, Martin Lutz, Anthony L. Spek, Koop Lammertsma, Chem. Commun., 2002, (4),348-349 doi:10.1039/b109816k
External links
- Magnus Walter's Selected Aspects of Organosilicon Chemistry
- Silicon in organic synthesis
- Safety data for methyltrichlorosilane from the Chemistry Department at Oxford University.
- S. Marsden (Editor): Contemporary organosilicon chemistry. Thematic Series in the Open Access Beilstein Journal of Organic Chemistry.
Categories:- Organosilicon compounds
Wikimedia Foundation. 2010.