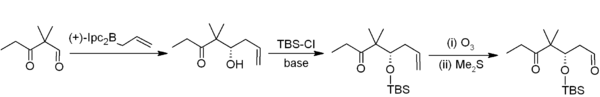- Organoboron chemistry
-
Organoborane or organoboron compounds are chemical compounds that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds.[1][2] Organoboron compounds are important reagents in organic chemistry enabling many chemical transformations, the most important one called hydroboration.
Contents
Properties
The C-B bond has low polarity (the difference in electronegativity 2.55 for carbon and 2.04 for boron) and therefore alkyl boron compounds are in general stable though easily oxidized. Vinyl groups and aryl groups donate electrons and make boron less electrophilic and the C-B bond gains some double bond character. Like the parent borane, diborane, organoboranes are classified in organic chemistry as strong electrophiles because boron is unable to gain a full octet of electrons. Unlike diborane however, organoboranes do not form dimers.
Other boranes of interest are carboranes, cluster compounds of carbon and boron and borabenzene, the boron equivalent of benzene.
Organoboranes with carbon replaced by oxygen are borinic esters R2BOR, boronic esters RB(OR)2 and borates B(OR)3 such as trimethylborate. In organometallic chemistry compounds with metal to boron bonds are called boryls (M–BR2) or borylenes (M–B(R)–M).
Synthesis
From Grignards
Simple organoboranes such as triethylborane or tris(pentafluorophenyl)boron can be prepared from trifluoroborane (as the ether complex) and the ethyl or pentafluorophenyl Grignard reagent.
From alkenes
Boranes react rapidly to alkenes in a process called hydroboration. This concept was discovered by Dr. Herbert Charles Brown at Purdue University, work for which he eventually received the Nobel Prize (jointly with Georg Wittig for his discovery of the Wittig reaction). Although diborane as a pure compound is a dimer, BH3 forms 1:1 complexes with basic solvents, for instance THF. In an ordinary electrophilic addition reaction of HX (X = Cl, Br, I, etc.) the Markovnikov's rule, which states that the lest electronegative atom, usually hydrogen adds to the least substituted carbon of the double bond, this determines regioselectivity. With boranes the mode of action is the same, the hydrogen adds to the most-substituted carbon because boron is least electronegative than hydrogen. The reason is that boron is less electronegative than hydrogen. When a positive charge develops in the alkene on the most substituted carbon atom, that is where the partially negatively charged hydrogen atom adds, leaving the least substituted carbon atom for the boron atom. The so called anti-Markovnikov addition because when the boron is replaced with a hydroxyl group the overall reaction is addition of water over the double bond in what appears to be an anti.Makovnikov addition.
This is most pronounced when the boron compound has very bulky substituents. One organoboron reagent that is often employed in synthesis is 9-borabicyclo[3.3.1]nonane or 9-BBN which is generated from the reaction of cyclooctadiene and diborane [3]. Hydroborations take place stereospecifically in a syn mode, that is on the same face of the alkene. In this concerted reaction the transition state is represented as a square with the corners occupied by carbon, carbon, hydrogen and boron with maximum overlap between the two olefin p-orbitals and the empty boron orbital.
Reactions
Hydroboration-oxidation
Main article: Reactions of organoborates and boranesIn organic synthesis the hydroboration reaction is taken further to generate other functional groups in the place of the boron group. The Hydroboration-oxidation reaction offers a route to alcohols by oxidation of the borane with hydrogen peroxide or to the carbonyl group with the stronger oxidizing agent chromium oxide.
Rearrangements
A second group of reactions that organoboron compounds are involved in create new carbon carbon bonds. Carbon monoxide is found to react very easily with a trialkylborane. What follows is a 1,2-rearrangement when an alkyl substituent on the anionic boron migrates to the adjacent electrophilic carbon of the carbonyl group. The carbonyl group can then be reduced to a hydroxyl group[citation needed].
Allylboration
Asymmetric allylboration demonstrates another useful application of organoboranes in carbon–carbon bond formation.[4] In this example from Nicolaou's synthesis of the epothilones,[5] asymmetric allylboration (using an allylborane derived from chiral alpha-pinene) is used in conjunction with TBS protection and ozonolysis. Overall, this provides a two-carbon homologation sequence that delivers the required acetogenin sequence.
Transmetallation
Organoboron compounds also lend themselves to transmetalation reactions with organopalladium compounds. This reaction type is exemplified in the Suzuki reaction.
As reducing agent
Borane hydrides such as 9-BBN and L-selectride (lithium tri-sec-butylborohydride) are reducing agents. An example of an asymmetric catalyst for carbonyl reductions is the CBS catalyst. This catalyst is also based on boron, the purpose of which is coordination to the carbonyl oxygen atom.
Borates
Trialkylboranes, BR3, can be oxidized to the corresponding borates, B(OR)3. One method for the determination of the amount of C-B bonds in a compound is by oxidation of R3B with trimethylamine oxide (Me3NO) to B(OR)3. The trimethylamine (Me3N) formed can then be titrated.
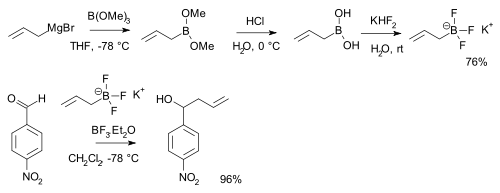
Boronic acids RB(OH)2 react with potassium bifluoride K[HF2] to form trifluoroborate salts K[RBF3][6] which are precursors to nucleophilic alkyl and aryl boron difluorides, ArBF2.[7] The salts are more stable than the boronic acids themselves and used for instance in alkylation of certain aldehydes:[8][note 1]
Boryllithium
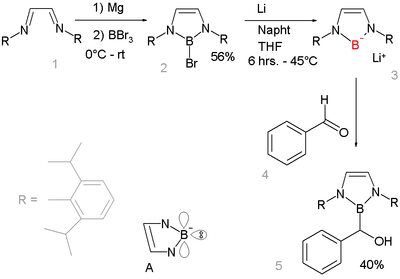
Nucleophilic anionic boryl compounds have long been elusive but a 2006 study described a boryllithium compound which reacts as a nucleophile [9][10]:
This is remarkable because in other period 2 elements lithium salts are common e.g. lithium fluoride, lithium hydroxide lithium amide and methyllithium. Reaction of base with a borohydride R2BH does not result in deprotonation to the boryl anion R2B- but to formation of the boryl anion R2B-H(base)+ because only this reaction path gives a complete octet [11]. Instead the boryl compound is prepared by reductive heterolysis of a boron-bromide bond by lithium metal. The new boryl lithium compound is very similar to and isoelectronic with N-heterocyclic carbenes. It is designed to benefit from aromatic stabilization (6-electron system counting the nitrogen lone pairs and an empty boron p-orbital, see structure A) and from kinetic stabilization from the bulky 2,6-diisopropylphenyl groups. X-ray diffraction confirms sp2 hybridization at boron and its nucleophilic addition reaction with benzaldehyde gives further proof of the proposed structure.
Alkylideneboranes
Alkylideneboranes of the type RB=CRR with a boron – carbon double bond are rarely encountered. An example is borabenzene. The parent compound is HB=CH2 which can be detected at low temperatures. A fairly stable derivative is CH3B=C(SiMe3)2 but is prone to cyclodimerisation [12].
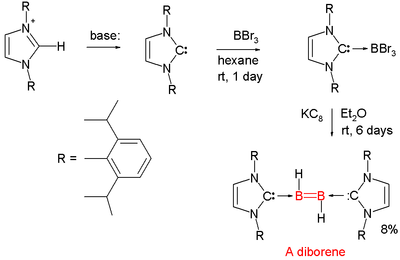
Diborenes
Chemical compounds with boron to boron double bonds are rare. In 2007 the first neutral diborene (RHB=BHR) was presented [13][14][15]. Each boron atom has a proton attached to it and each boron atom is coordinated to a so-called NHC carbene.
Boroles
The cyclic compound borole, a structural analog of pyrrole, has not been isolated, but substituted derivatives known as boroles are known.
Other uses
TEB – Triethylborane was used to ignite the JP-7 fuel of the Pratt / Whitney J-58 ramjet engines powering the Lockheed SR-71 Blackbird.
Notes
- ^ Displayed is a reaction sequence starting with reaction of allyl magnesium bromide with trimethyl borate, followed by hydrolysis of the boronic ester to the boronic acid with hydrochloric acid. The aldehyde is p-nitrobenzaldehyde.
See also
- Compounds of carbon with other elements in the periodic table:
CH He CLi CBe CB CC CN CO CF Ne CNa CMg CAl CSi CP CS CCl CAr CK CCa CSc CTi CV CCr CMn CFe CCo CNi CCu CZn CGa CGe CAs CSe CBr CKr CRb CSr CY CZr CNb CMo CTc CRu CRh CPd CAg CCd CIn CSn CSb CTe CI CXe CCs CBa CHf CTa CW CRe COs CIr CPt CAu CHg CTl CPb CBi CPo CAt Rn Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Uuq Uup Uuh Uus Uuo ↓ CLa CCe CPr CNd CPm CSm CEu CGd CTb CDy CHo CEr CTm CYb CLu Ac Th Pa CU Np Pu Am Cm Bk Cf Es Fm Md No Lr Chemical bonds to carbon Core organic chemistry Many uses in chemistry Academic research, but no widespread use Bond unknown / not assessed References
- ^ The Roles of Boron and Silicon, Susan E. Thomas; Oxford Chemistry Primers No.1; 1991: Very good general book covering all the important reactions of boron and organoboranes in organic chemistry.
- ^ Organometallics Christoph Elschenbroich 3rd Ed. 2006 ISBN 3-527-29390-6 – Wiley-VCH, Weinheim
- ^ Advanced Organic Chemistry, F.A. carey, R.J. Sundberg ISBN 0-306-41088-5
- ^ Lachance, H.; Hall, D. Org. React. 2008, 73, 1. (doi: 10.1002/0471264180.or073.01)
- ^ Nicolaou, K.C.; Sarabia, F.; Ninkovic, S.; Finlay, M.R.V.; Boddy, C.N.C. (1998). "Probing The Ring Size Of Epothilones: Total Synthesis Of 14-, 15-, 17-, And 18 Epothilones A". Angewandte Chemie. International edition in English 37 (1–2): 81–84. http://cat.inist.fr/?aModele=afficheN&cpsidt=10349604. Retrieved 2008-03-02.
- ^ Conversion of Arylboronic Acids into Potassium Aryltrifluoroborates: Convenient Precursors of Arylboron Difluoride Lewis Acids E. Vedejs, R. W. Chapman, S. C. Fields, S. Lin, M. R. Schrimpf J. Org. Chem. 1995; 60(10); 3020–3027. doi:10.1021/jo00115a016
- ^ Organotrifluoroborates and Monocoordinated Palladium Complexes as Catalysts—A Perfect Combination for Suzuki–Miyaura Coupling Gary A. Molander and Belgin Canturk Angew. Chem. Int. Ed. 2009, 48, 9240 – 9261doi:10.1002/anie.200904306
- ^ Organoboron compounds as mild nucleophiles in Lewis acid- and transition metal-catalyzed C–C bond-forming reactions Robert A. Batey, Tan D. Quach, Ming Shen, Avinash N. Thadani, David V. Smil, Sze-Wan Li, and D. Bruce MacKay Pure Appl. Chem., Vol. 74, No. 1, pp. 43–55, 2002. http://www.iupac.org/publications/pac/2002/pdf/7401x0043.pdf
- ^ Boryllithium: Isolation, Characterization, and Reactivity as a Boryl Anion Yasutomo Segawa, Makoto Yamashita, Kyoko Nozaki Science 6 October 2006: Vol. 314. no. 5796, pp. 113 – 115 doi:10.1126/science.1131914
- ^ Boron Attacks Electropositive element pressed into action as nucleophilic boryllithium Bethany Halford Chemical & Engineering News October 9, 2006 Volume 84, Number 41 p. 11 Link
- ^ Boronic Acids: Preparation, Applications in Organic Synthesis and Medicine. Dennis G. Hall ISBN 3-527-30991-8
- ^ Reactions at the Boron-Carbon Double Bond of Methyl(methylidene)boranes Peter Paetzold, Ulli Englert, Rudolf Finger, Thomas Schmitz, Alexander Tapper, and Ralf Ziembinski Z. Anorg. Allg. Chem. 2004, 630, 508–518 doi:10.1002/zaac.200300396
- ^ A Stable Neutral Diborene Containing a BdB Double Bond Yuzhong Wang, Brandon Quillian, Pingrong Wei, Chaitanya S. Wannere, Yaoming Xie, R. Bruce King, Henry F. Schaefer, III, Paul v. R. Schleyer, and Gregory H. Robinson doi:10.1021/ja075932i
- ^ Neutral Diborene Is A First Ron Dagani Chemical & Engineering News October 1, 2007 Volume 85, Number 40 p. 10 [1]
- ^ The boron precursor is Boron tribromide and the reducing agent is KC8 which abstracts the required protons from diethyl ether solvent.
Categories:- Organoboron compounds
Wikimedia Foundation. 2010.